Documente Academic
Documente Profesional
Documente Cultură
Chapter 11 Carbon Compound
Încărcat de
vaoger0 evaluări0% au considerat acest document util (0 voturi)
181 vizualizări50 paginiSimple note
Drepturi de autor
© Attribution Non-Commercial (BY-NC)
Formate disponibile
DOCX, PDF, TXT sau citiți online pe Scribd
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentSimple note
Drepturi de autor:
Attribution Non-Commercial (BY-NC)
Formate disponibile
Descărcați ca DOCX, PDF, TXT sau citiți online pe Scribd
0 evaluări0% au considerat acest document util (0 voturi)
181 vizualizări50 paginiChapter 11 Carbon Compound
Încărcat de
vaogerSimple note
Drepturi de autor:
Attribution Non-Commercial (BY-NC)
Formate disponibile
Descărcați ca DOCX, PDF, TXT sau citiți online pe Scribd
Sunteți pe pagina 1din 50
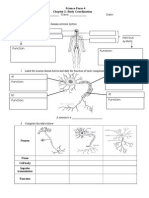
4541 CHEMISTRY SPM Chapter 11
Chapter 11 Carbon Compounds
1
CHAPTER 11 : CARBON COMPOUND
11.1 CARBON COMPOUNDS
1. Carbon compounds are compounds that contain the .. element.
These compounds can be classified into two groups :
(a) Organic compounds
(b) Inorganic compounds
2. Organic compounds carbon compounds that are obtained from ...
(plants and animals) such as sugar/glucose (C
6
H
12
O
6
), starch, protein, vitamin, enzyme etc.
3. Inorganic compounds carbon compounds that are usually do not contain carbon to
carbon bonds such as carbon monoxide (CO), carbon dioxide (CO
2
), calsium carbonate
(CaCO
3
) etc.
4. Most organic compounds contain the element and .
Complete combustion of organic compounds produces carbon dioxide and water.
Activity 1:-
1. Complete the mind map below:
2. Group these following carbon compounds into organic compounds and inorganic compounds.
Name Formula Name Formula
Calcium carbonate
CaCO
3
Potassium cyanide
KCN
Urea
(NH
2
)
2
CO
Amino acid
NH
2
CH(CH
3
)COOH
Carbon dioxide
CO
2
Methane
CH
4
Glucose
C
6
H
12
O
6
Butanol
C
4
H
9
OH
Ethanoic acid
CH
3
COOH
Sodium bicarbonate
NaHCO
3
Organic Compounds Inorganic Compounds
Carbon compound
4541 CHEMISTRY SPM Chapter 11
Chapter 11 Carbon Compounds
2
11.2 A : HYDROCARBON
1. Hydrocarbons are organic compounds that contain only carbon, C and hydrogen, H.
2. Hydrocarbons are classified into two groups :
(a) Saturated hydrocarbons contain only single covalent bonds between carbon atoms.
Example :
(b) Unsaturated hydrocarbon contains at least one double or triple covalent bond between
carbon atoms.
Example :
3. The main source of hydrocarbons is . It is formed as a result of
decomposition of plants and animals that died million years ago.
4. . is a mixture of different molecular size hydrocarbons. These hydrocarbons
can be separated using of petroleum at different temperature in
an oil refinery. This process separates hydrocarbons with different molecular size depends on
the boiling points of the hydrocarbons. ( eg : petroleum gas, petrol, naphta, kerosene, diesel,
lubricating oil and diesel).
H H H
H C C C H
H H
H C H
H
H H H H
H C C C C H
H H H H
Single covalent bonds between carbon atoms
Double covalent bond
between carbon atoms
H H H
H C C C H
H
Triple covalent bond
between carbon atoms
H
H C C C H
H
4541 CHEMISTRY SPM Chapter 11
Chapter 11 Carbon Compounds
3
11.3 ALKANE
1. It is classified as saturated hydrocarbons. (A saturated hydrocarbon contains only single
covalent bonds between carbon atoms).
2. Alkanes are hydrocarbons with the general formula :
C
n
H
2n + 2
where n = 1, 2, 3, ,
3. Each carbon atom is bonded to four other atoms by single covalent bonds that is C C.
Example : Formation of methane molecule
Methane, CH
4
H
H C H
H
- - x and represents one pair of electron form a single covalent bond.
- *Structural formula a chemical formula that shows how atoms are bonded to
each other covalently in a molecule.
- A carbon atom with proton number 6 and its electron arrangement is 2.4 must have a
total of four covalent bonds ( sharing 4 pairs of electron to achieve octet electron
arrangement)
each carbon atom must have four in its structural molecule.
C
H
H
H
H
*Structural formula
Molecular formula
Name
4541 CHEMISTRY SPM Chapter 11
Chapter 11 Carbon Compounds
4
Activity 2 :-
Draw the structural formula for ethane, C
2
H
6
; propane, C
3
H
8
; butane, C
4
H
10
;
pentane, C
5
H
12
; hexane, C
6
H
14
and heptane, C
7
H
16
.
4. Naming of Alkanes
(a) The names of straight chain alkanes (all the carbon atoms are joined in a continuous
chain) are made up of two component parts :
(i) Stem / root
indicates the number of carbon atoms in the longest continuous carbon chain.
The names of stems for the first ten straight alkanes are :
Number of
carbon atom
1 2 3 4 5 6 7 8 9 10
Stem Meth Eth Prop But Pent Hex Hept Oct Non Dec
(ii) Suffix / ending
indicates the group (homologous series) of the compound.
For alkane, the suffix is ane because it belongs to the alkane group.
4541 CHEMISTRY SPM Chapter 11
Chapter 11 Carbon Compounds
5
Activity 3 :-
Complete the table below :
Number of
carbon
atoms,
n
Molecular
Formula,
C
n
H
2n+2
Structural Formula Name of alkane
1
CH
4
methane
2
C
2
H
6
ethane
3
C
3
H
8
4
5
C
5
H
12
pentane
6
7
C
7
H
16
heptane
8
C
8
H
18
octane
9
10 decane
4541 CHEMISTRY SPM Chapter 11
Chapter 11 Carbon Compounds
6
5. Physical properties of alkanes
(a) Alkanes are molecular compounds (covalent compound) which consist of molecules.
The atoms in the molecules are bonded together by a strong covalent bond.
These molecules are held together by weak van der Waals forces (intermolecular forces).
(b) Alkenes have physical properties similar to other covalent compounds.
Alkenes are insoluble in water but dissolve in organic solvents, cannot conduct electricity,
low melting and boiling points and less dense than water.
Activity 4 :-
Complete the following table :
Name of
members
Molecular
Formula
Molar mass/
g mol
-1
Melting
point/C
Boiling
point/C
Physical state at
room temperature
25C
Methane CH
4
16 -182 -162
Ethane 30 -183 -89
Propane 44 -188 -42
Butane C
4
H
10
58 -138 -0.5
Pentane 72 -130 36
Hexane 86 -95 69
Heptane 100 -91 98
Octane C
8
H
18
114 -57 126
Nonane -54 153
Decane -30 174
The first . members of alkanes exist as . at room temperature. Pentane to
decane are .
As the number of carbon atoms in a molecule of alkane increases :
The molecular size of alkane ., the intermolecular forces becomes
., more . energy is needed to . this forces,
the melting and boiling points .
The viscosity and density of alkane .
The alkane become . to ignite.
Hydrogen atom
Carbon atom
Methane molecule
Covalent bond //
Intramolecular force
van der Waals force //
Intermolecular force
4541 CHEMISTRY SPM Chapter 11
Chapter 11 Carbon Compounds
7
6. Chemical properties of alkanes
(a) Alkanes only have single covalent bonds, C C and C H . They are saturated
hydrocarbon and chemically not reactive.
(b) Two chemical reactions of alkanes are :
I : Combustion Reaction
- Alkanes undergo complete combustion in the presence of sufficient/excess
oxygen to form carbon dioxide and water only.
Alkane + oxygen carbon dioxide + water
Activity 5 :-
Balance the equations :
(i) CH
4
+ O
2
CO
2
+ H
2
O
(ii) C
2
H
6
+ O
2
CO
2
+ H
2
O
(iii) C
3
H
8
+ O
2
CO
2
+ H
2
O
- Alkanes undergo incomplete combustion when there is insufficient oxygen to
form carbon dioxide gas, carbon monoxide gas, carbon (in the form of soot) and
water.
Example :
2CH
4
+ 3O
2
C + CO
2
+ 4H
2
O
or
2CH
4
+ 3O
2
2C + 2CO + 2CO
2
+ 12H
2
O
- When alkanes are burnt, large quantities of heat energy are released. This makes
alkanes suitable for use as a fuel.
II : Substitution Reaction
Occurs when an alkane is mixed with a halogen in the presence of sunlight (ultraviolet
light, u.v).
In this reaction, each hydrogen atoms in the alkane molecule are substituted one by one
by halogen atoms.
Example :
When methane reacts with chlorine gas in the presence of ultraviolet light, a variety of
substituted products are formed :
CH
4
+ Cl
2
CH
3
Cl + HCl
(methane) (chloromethane)
CH
3
Cl + Cl
2
CH
2
Cl
2
+ HCl
(dichloromethane)
CH
2
Cl
2
+ Cl
2
+ HCl
(trichloromethane)
CHCl
3
+ Cl
2
+ HCl
( tetrachloromethane)
Tips! (Balancing the equations)
Step 1 : Balance C
Step 2 : Balance H
Step 3 : Balance O
(can use friction)
UV
UV
UV
UV
4541 CHEMISTRY SPM Chapter 11
Chapter 11 Carbon Compounds
8
(c) The uses of methane in everyday life :
Methane is the major component in natural gas (gas found together with
petroleum).
Methane gas is produced when waste organic matter decompose in the absence of
oxygen. As methane is a combustible gas, it can cause fire in landfills and pit
swamps.
11.4 ALKENE
1. It is classified as unsaturated hydrocarbons.
(An unsaturated hydrocarbon contains at least one double covalent bonds between
carbon atoms).
2. Alkenes are hydrocarbons with the general formula :
C
n
H
2n
where n = 2, 3, ,
3. Every alkene has a carbon-carbon double covalent bond, C = C in its molecule.
Example : Formation of an ethene molecule
(a) Ethene, C
2
H
4
H H
H
C C H
Structural formula for ethane, C
2
H
4
* The first member of alkenes has two carbon atoms in a molecule because
. bond is formed between two carbon atoms.
4. Naming of Alkenes
(a) The name of straight chain alkenes are also made up of two component parts :
(i) Stem/root : indicates the number of carbon atoms in the longest continuous carbon
chain.
The names of stems for the first nine straight alkanes are :
eth, prop, but, pent, hex, hept, oct, non and dec
(ii) Suffix/ ending : indicates the group of the compound.
For alkene, the suffix is ene because it belongs to the alkene group.
(b) Naming the straight chain alkene :
(i) Determine the longest carbon chain containing double bond.
give the stem name according to the number of carbon atoms ;
eth, prop, but, pent, hex, hept, oct, non and dec
(ii) Add the suffix ene at the end of the name.
(iii) Number the carbon atoms from the end nearer to the double bond and give the
double bond the smaller number.
H
C
C
H
H
H
4541 CHEMISTRY SPM Chapter 11
Chapter 11 Carbon Compounds
9
Activity 6 :-
Complete the table below :
Number of
carbon
atoms,
n
Molecular
Formula,
C
n
H
2n
Structural Formula Name of alkane
2
C
2
H
4
ethene
3
4
5
6
7
8
9
10
C C
H H
H H
4541 CHEMISTRY SPM Chapter 11
Chapter 11 Carbon Compounds
10
5. Physical properties of Alkenes are similar to alkanes.
Activity 7 :-
Complete the following table :
Name of
members
Molecular
Formula
Molar mass/
g mol
-1
Melting
point/ C
Melting
point /
o
C
Boiling
point/ C
Boiling
point /
o
C
Density
Ethene C
2
H
4
28 -169
-104
Propene -185 -47
But-1-ene -185 -6
Pent-1-ene -165 30
Heks-1-ene -140 63
Hept-1-ene -119 93
Oct-1-ene -104 122
Non-1ene -94 146
Dec-1-ene 87 171
Physical state at room temperature (25
O
C) :
Ethene, propene and but-1-ene are ..
Pent-1-ene to non-1-ene are .. and dec-1-ene is ..
Alkenes have .. melting and boiling points because the ..
van der Waals forces (intermolecular forces) between small molecules need
amount of heat energy to overcome the forces.
As the number of carbon atoms per molecule increases, the molecular size of alkane
., the .. the intermolecular forces, more ..
energy is needed to .. this forces, the melting and boiling points
..
4541 CHEMISTRY SPM Chapter 11
Chapter 11 Carbon Compounds
11
6. Chemical properties of Alkenes
(a) Alkenes are chemically .. reactive than alkanes because of the existence of
double covalent bond between two carbon atoms.
Almost all of the chemical reactions of alkene occur at the double bond.
(b) The chemical reactions of alkenes are :
I : Combustion Reaction
Alkene burns completely in the excess oxygen to produce . and
Alkene + O
2
CO
2
+ H
2
O
Activity 8 :-
1 Balance the following equations :
(i) C
2
H
4
+ O
2
CO
2
+ H
2
O
(ii) C
3
H
6
+ O
2
CO
2
+ H
2
O
(iii) C
4
H
8
+ O
2
CO
2
+ H
2
O
2 Write the balanced equation for combustion reaction of :
(i) Pentene : ..
(ii) Hexene : ..
(iii) Octene: ..
However, alkene burns incompletely (in limited supply of oxygen) to form carbon dioxide,
carbon monoxide, carbon (in the form of soot) and water.
Example :
2C
2
H
4
+ 3O
2
2C + 2CO + 2H
2
O
or
C
2
H
4
+ 2O
2
2CO + 2H
2
O
4541 CHEMISTRY SPM Chapter 11
Chapter 11 Carbon Compounds
12
II : Addition Reaction
v As alkenes are unsaturated hydrocarbon, they undergo addition reaction.
An addition reaction is a reaction in which other atoms are added to each carbon atom of the
.. bond, [C = C ] to form .. covalent bond product [ C - C ].
C = C + XY C C
X Y
(unsaturated) (saturated )
v Simple molecules like hydrogen, H
2
; hydrogen chloride, HCl ; water, H
2
O or halogens, [F
2
,
Cl
2
, Br
2
, I
2
] can be added to the double bond.
(i) Addition of Hydrogen (Hydrogenation)
- Alkenes react with hydrogen at 180 C in the presence of nickel/platinum as
a catalyst to produce alkanes.
- Hydrogenation is used to prepare an alkane (saturated compound) from an
alkene (unsaturated compound) in industry.
Example :
H H H H
H C C H + H
2
H C C H
Ethene H H
Ethane
(ii) Addition of Halogen (Halogenation)
- Alkenes react with halogens such as chlorine,Cl
2
and bromine, Br
2
at room conditions.
(no catalyst or ultraviolet light needed)
Example :
H H H H
H C C H + Br
2
H C C H
Ethene (bromine water) Br Br
1,2-dibromoethane
- When ethene gas is passed through bromine water, brown colour of bromine water is
decolourised // colourless solution formed.
- This reaction is used to distiguish a saturated hydrocarbon and unsaturated hydrocarbon.
Ni / Pt
180 C
C
2
H
4
gas
Bromine
water
4541 CHEMISTRY SPM Chapter 11
Chapter 11 Carbon Compounds
13
(iii) Addition of Hydrogen Halide [ HX HCl, HBr, HI ]
- Alkenes react with hydrogen halide such as hydrogen chloride, HCl or hydrogen
bromide, HBr at room temperature to form haloalkane.
Example :
C
2
H
4
+ HCl C
2
H
5
Cl
Ethene Hydrogen chloride Chloroethane
C
3
H
6
+ HBr
Propene Hydrogen bromide .
Activity 9 :-
Complete the equation and draw the structural formulae for all the reactants and products in the
equation below :
1 (a) C
3
H
6
+ H
2
C
3
H
8
(b) C
4
H
8
+ H
2
.
(c) . + H
2
C
6
H
14
2 (a) C
3
H
6
+ Br
2
C
3
H
6
Br
2
(b) C
4
H
8
+ Cl
2
.
(c) . + I
2
C
6
H
12
I
2
4541 CHEMISTRY SPM Chapter 11
Chapter 11 Carbon Compounds
14
(iv) Addition of Oxidizing agent : (Oxidation reaction)
Acidified Potassium manganate(VII), KMnO
4
solution
Acidified Potassium dichroromate(VI), K
2
Cr
2
O
7
solution
In this reaction, two hydroxyl, -OH groups are added to the carbon-carbon
in an alkene molecule.
An alkene the colour of acidified potassium
manganate(VII) solution // the orange solution of acidified Potassium
dicrhoromate(VI), K
2
Cr
2
O
7
turns to
This reaction is used to distiguish a hydrocarbon and
hydrocarbon.
Example :
C
2
H
4
+ H
2
O + [O] C
2
H
4
(OH)
2
Ethene Ethan-1,2-diol
Activity 10 :-
Complete the following equation :
(a) C
3
H
6
+ H
2
O + [O] C
3
H
6
(OH)
2
H H
(b) H C = C H + H
2
O + [O]
(v) Addition of Water (Hydration)
v Alkenes react with water (in the form of steam) at high temperature and pressure in the
presence of phosphoric acid as a catalyst to produce alchohols.
Alkene + H
2
O Alcohol
Example :
C
2
H
4
+ H
2
O C
2
H
5
OH
Ethene ethanol
H
3
PO
4
300 C / 60 atm
H
3
PO
4
300 C / 60 atm
KMnO
4
(aq)
[Purple]
Alkene
Colourless
C
2
H
6
gas
KMnO
4
C
H
H C
H
H
OH OH
Propan-1,2-diol
Ethan-1,2-diol
4541 CHEMISTRY SPM Chapter 11
Chapter 11 Carbon Compounds
15
Activity 11 :-
Complete the following equation :
C
3
H
6
+ H
2
O
H H
H C = C H + H
2
O
v Addition of steam to alkene is one way to manufacture alcohol in industry :
C
n
H
2n
+ H
2
O(g) C
n
H
2n+1
OH
Alkene Alcohol
Example :
C
4
H
8
+ H
2
O
Butene
(vi) Addition Polymerisation reaction
+ In this reaction, small alkene molecules undergo addition reaction at a high pressure of 1000
atm and temperature of 200 C. Thousands of alkene molecule join together to form long
chain giant molecules called
+ The small repeating units of molecules that join together to form polymer are called
Example : Polymerisation of ethene
Polythene
H
3
PO
4
300 C / 60 atm
H
3
PO
4
300 C / 60 atm
H H
H C C H
Ethene
n
H H
C C
H H
n is large number up
to a few thousands
n
H
3
PO
4
300 C / 60 atm
H
3
PO
4
300 C / 60 atm
4541 CHEMISTRY SPM Chapter 11
Chapter 11 Carbon Compounds
16
Activity 12 :-
Polymerisation of propene, C
3
H
6
Polymerisation of butane, C
4
H
8
n
H H H
H C C C H
H
propene
n
H H H H
H C C C C H
H H
butene
4541 CHEMISTRY SPM Chapter 11
Chapter 11 Carbon Compounds
17
11.5 COMPARING PROPERTIES OF ALKANE WITH ALKENE (Using hexane and hexene in the laboratory)
Chemical
properties
Procedure
Observation Explanation/Chemical Equation for
reaction Hexane Hexene
Sootiness of flame
4541 CHEMISTRY SPM Chapter 11
Chapter 11 Carbon Compounds
18
Chemical
properties
Procedure
Observation Explanation/Chemical Equation for
reaction Hexane Hexene
Reaction with
bromine water
4541 CHEMISTRY SPM Chapter 11
Chapter 11 Carbon Compounds
19
Chemical
properties
Procedure
Observation Explanation/Chemical Equation for
reaction Hexane Hexene
Reaction with
acidified
potassium
manganate(VII)
solution
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
20
11.6 Homologous Series
1. Homologous series are groups of carbon compounds that have the following general characteristics :
(a) Members having the same properties because they have the same
. (group that takes part in a reaction).
(b) Members of the series can be represented by a formula.
(c) Members of the series can be prepared by the method.
(d) Two consecutive members in the series have a difference in relative atomic mass of 14,
a difference of CH
2
.
(e) Members of the series have physical properties that gradually as the
number of carbon atoms and hydrogen atoms in a molecule
2. Example of homologous series :
Homologous Series General Formula
Functional
group
Type of carbon
compound
Alkane
C
n
H
2n+2
n = 1, 2, 3,
C C
Single bond
Saturated hydrocarbon
Alkene
C
n
H
2n
n = 2, 3, 4,
C = C
Double bond
Unsaturated hydrocarbon
Alcohol
C
n
H
2n+1
OH
n = 1, 2, 3,
-OH
Hydroxyl
group
Non-hydrocarbon
Carboxylic acid
C
n
H
2n+1
COOH
n = 0, 1, 2, 3,
-COOH
Carboxyl
group
Non-hydrocarbon
Ester
C
n
H
2n+1
COO C
n
H
2n+1
n = 0, 1, 2, 3,
n = 1, 2, 3,
-COOC-
Non-hydrocarbon
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
21
11.7 NAMING ALKANE AND ALKENE USING IUPAC NOMENCLATURE
1. Three parts in the naming of alkane and alkene.
(a) Prefix shows the branch group (alkyl group) with general formula C
n
H
2n + 1,
attached to the longest carbon chain.
H
|
H C H
|
methyl
(b) Stem shows the number of carbon atom in the longest carbon chain.
Number of
carbon
atom
1 2 3 4 5 6 7 8 9 10
Stem Meth Eth Prop But Pent Hex Hept Oct Non Dec
(c) Suffix shows shows the Homolog Series.
- Alkane : ane
- Alkene : ene
- Alcohol : ol
- Carboxylic acid : oic
2. Steps in naming alkanes and alkenes
S1: Identify the funtional group /homologous series of the compound. gives the name of the suffix
Single bond - Alkane - ane
Double bond - Alkene - ene
S2 : Identify the longest carbon chain, the number of carbon atoms in the longest carbon chain gives
the name of the stem e.g : meth, eth, prop, but, pent
S3: Identify the branch chain. Determine the prefix and numbered the carbon atoms in the longest
carbon chain beginning from the end of the chain nearer to the branch chain. The name
for the branch chain ends with yl
Number
of carbon
Name of
branch chain
1 Methyl
2 Ethyl
3 Prophyl
4 Butyl
5 Pentyl
6 Hexyl
H H
| |
H C C
| |
H H
ethyl
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
22
Number of carbon
in the longest chain
(4 carbon atoms)
Homologous series
(alkene)
3. Method of writing the Name of organic compound using IUPAC nomenclature
Prefix
(Branch)
Stem
( number of carbon atoms in the
longest carbon chain)*
Suffix
(functional group / homologous series)
* for alkenes, the smallest number is given to the carbon with the double bond.
Example :
2,3-dimethylbut-1-ene
Activity 14 :-
(a) State the name the following compounds.
(i)
CH
3
|
H
3
C CH CH
2
CH
2
CH
3
Name : ..
(ii)
H
3
C CH CH
2
CH
2
CH
3
|
CH
2
CH
2
CH
2
CH
3
Name : ..
(iii)
CH
3
|
H
3
C CH = CH CH CH
3
Name : ..
name and name write close together
number and name, write -
number and number, write ,
Suffix Stem
Branch
Prefix
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
23
(b) Draw the structural formula for the following molecules :
(i) 2,4-dimethyloctane
(ii) 2,2-dimethylhexane
(iii) 3,3,4-trimethylhexane
(iv) 4-methylpent-2-ene
(v) 4,5-dimethylhex-2-ene
(vi) 3-ethylpen-2-ene
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
24
11.8 ISOMERISM
1. (a) Molecular formula shows the type and number of atoms in a molecular compound.
(b) Structural formula shows the type and number of atoms for each element, and how the atoms are
bonded to one another in a compound.
2. Isomerism : phenomenon where a compound has the same molecular formula but different
structural formulae.
Activity 15 :-
Complete the following table :
Molecular
Formula
Structural Formula & IUPAC name
Number of
structural
formulae
Number
of
Isomers
CH
4
methane
C
2
H
6
ethane
C
3
H
8
propane
C
4
H
10
butane
C
5
H
12
pentane
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
25
Molecular
Formula
Structural Formula & IUPAC name
Number of
structural
formulae
Number
of
Isomers
C
2
H
4
ethene
C
3
H
6
propene
C
4
H
8
butene
C
5
H
10
pentene
*The isomers have similar chemical properties but different physical properties.
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
26
B : NON-HYDROCARBON
11.8 ALCOHOL
1. Alcohols are organic compounds that contain carbon, hydrogen and oxygen atoms.
2. The general formula for alcohol is :
C
n
H
2n + 1
OH
where n = 1, 2, 3, ,
3. Each member of alcohol series contains hydroxyl as the functional group ( -OH ) which is
covalently bonded to the carbon atom.
4. Naming alcohol using IUPAC nomenclature :
S1 : Determine the number of carbon atoms in the longest carbon chain which contains the
hydroxyl group ( OH ) obtain the name of alkane with the same number of carbon
atom as alcohol.
S2 : Replace the ending e from the name of alkane with ol
Example :
Methane Methanol
Ethane Ethanol
Propane Propanol
Butane Butanol
Penthane Pentanol
S3 : Number the carbon atom in the longest carbon chain which is joined to the hydroxyl
group -OH with the smallest number the number is placed in front of the ol to
indicate which carbon atom the hydroxyl group is attached to.
Example :
Butan-2-ol
the -OH is attached at the second carbon atom from the end.
The structural formula :
H H H H H H H H
H C C C C OH or H C C C C H
H H H H H H OH H
S4 : For alcohols with branches, write the names of all the branches as prefix.
H H H CH
3
H
H C C C C C H
H OH H H H
4 carbon atoms
-OH
-OH at the 2
nd
C
Butan-1-ol
C
4
H
9
OH
Butan-2-ol
C
4
H
9
OH
Isomer
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
27
5. Isomerism in alcohol :
Isomers are molecules that have same molecular formula but different structural formula.
Activity 13 :-
Complete the following table :
Name of
alcohol
Molecular
Formula
Condensed
structural formula
Structural Formula &
IUPAC name
Number of
isomers
Methanol CH
3
OH 0
Ethanol C
2
H
5
OH CH
3
CH
2
OH 0
Propanol C
3
H
7
OH
Propan-1-ol
2
Propan-2-ol
Butanol 4
Name : 4-methylpentan-2-ol
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
28
Methanol and ethanol do not have isomers because each molecule only has one structural formula.
Isomerism in alcohol begins with propanol.
6. Preparation of Ethanol
Two methods of preparation of Ethanol
(i) Preparation of ethanol, C
2
H
5
OH in the laboratory ( Fermentation of glucose)
Fermentation is a process in which microorganism such as yeast act on carbohydrates
(sugar or starch) to produce ethanol and cabon dioxide.
Yeast is added to glucose solution ( or fruit juices such as grape/pineapple juice) and left
in a warm place for a three days in the absence of oxygen.
Yeast contains enzyme (zymase) which break down the sugar/starch into glucose
(hydrolisis process) and then to ethanol and cabon dioxide.
Fermentation equation :
C
6
H
12
O
6
2C
2
H
5
OH + 2CO
2
Glucose Ethanol
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
29
The ethanol is purified by fractional distillation. (78
0
C)
(ii) Industrial production of ethanol, C
2
H
5
OH ( Hydration of ethene)
- Alkene is reacted with steam (H
2
O) at temperature; 300
o
C and pressure; 60 atm in
presence of phosphoric acid (H
3
PO
4
) as a catalyst.
- Chemical equation of the reaction :
C
2
H
4
+ H
2
O C
2
H
5
OH
Ethene steam ethanol
7. Physical properties of alcohol
1 Alcohols with one to eleven carbon atoms exist as liquids.
2 Methanol, ethanol and propanol are miscible in all proportions with water.
The solubility of alcohol in water decreases with increasing of their molecular size //
number of carbon atom.
3 Physical properties of ethanol, C
2
H
5
OH :
- colourless liquid
- mixes with water in all proportions
- less dense than water
- boiling point is 78
o
C at 1 atm.
8. Chemical properties of ethanol, C
2
H
5
OH
(a) Combustion of ethanol
(i) Ethanol burns in air to produce water and carbon dioxide. Ethanol burns readily with a
blue flame and without producing soot.
C
2
H
5
OH + 3O
2
2CO
2
+ 3H
2
O
(ii) Combustion of ethanol releases large amount of heat (ethanol is suitable for use as a
fuel)
Activity 13 :-
Balance the following equations :
2C
3
H
7
OH + 9O
2
6CO
2
+ 8H
2
O
C
4
H
9
OH + O
2
CO
2
+ H
2
O
C
5
H
11
OH + O
2
CO
2
+ H
2
O
C
6
H
13
OH + 9O
2
6CO
2
+ 7H
2
O
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
30
(b) Oxidation of ethanol
(i) Ethanol is oxidised by an oxidising agent such as acidified potassium
manganate(VII), KMnO
4
or acidified potassium dichromate(VI), K
2
Cr
2
O
7
solution.
(ii) Both these oxidising agents are represented as 2[O] in the chemical equation.
(iii) One oxygen atom joins the ethanol molecule to form C = O and the other oxygen
atom is joined to the two hydrogen atoms that are removed from the ethanol to form
H
2
O.
(iv) Oxidation of alcohol is the process where an alcohol molecule loses two H atoms
and receives one O atom.
Example :
C
2
H
5
OH + 2[O] CH
3
COOH + H
2
O
Ethanol Ethanoic acid water
or
H H H
H C C H + 2[O] H C C + H
2
O
H OH H
Activity 14 :-
Complete the following equations :
C
3
H
7
OH + 2[O] C
2
H
5
COOH + H
2
O
or
CH
3
CH
2
CH
2
OH + 2[O] + H
2
O
Propanol propanoic acid
or
H H H
H C C C H + 2[O] + H
2
O
H H OH
..
O
OH
From oxidising agent
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
31
(v) Alcohol undergoes oxidation reaction to produce carboxylic acid.
C
n
H
2n+1
+ 2[O] C
n
H
2n+1
COOH + H
2
O
This reaction is used to prepare carboxylic acid.
(c) Dehydration of ethanol
(i) Dehydration of an alcohol involves the removal of water molecule from each of
alcohol molecule.
(ii) Water molecule from the alcohol molecule is removed by a dehydrating agents
such as porcelain chips / aluminium oxide (alumina) / concentrated sulphuric acid /
concentrated phosphoric acid.
(iii) There are two ways to carry out the dehydration of ethanol in the laboratory :
1 Hot vapour of ethanol is passed over a heated catalyst such as porcelain chips
/aluminium oxide.
2 Ethanol is heated under reflux with excess of concentrated sulphuric acid /
concentrated phosphoric acid.
Draw a labeled diagram to show the set-up of apparatus to carry out the
dehydration of ethanol.
(iv) Dehydration of ethanol produces ethene and water.
Equation for the reaction :
H H H H
porcelain
H C C H H C C H + H
2
O
n = n - 1
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
32
H OH
or
C
2
H
5
OH C
2
H
4
+ H
2
O
Ethanol Ethene
Activity 15 :-
Complete the following equations :
(a) C
3
H
7
OH + H
2
O
..
(b) + H
2
O
Butanol
(c) + H
2
O
(d) C
n
H
2n+1
OH C
n
H
2n
+ H
2
O
n = 2, 3, 4..
Dehydration of alcohol produces .................................
9. Uses of alcohol
(a) As a fuel combustion of alcohol produces water and carbon dioxide (clean emission) and releases
a lot of heat energy (exothermic).
Example :
CH
3
OH + O
2
CO
2
+ 2H
2
O H = - x kJ mol
-1
(b) As a solvent to dissolve many organic compound such as paint, varnish, lacquer, and perfume.
(c) In medical field as a solvent for the preparation of certain medicines. Ethanol for example is used
as an antiseptic and an ingredient in cough mixtures.
(d) Production of cosmetics main component in production of cosmetics, creams, lotions, soaps and
others.
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
33
10. Alcohol Misuse and Abuse
Activity 16 :-
Complete the following chart :
2.9 CARBOXYLIC ACID [ ORGANIC ACID ]
1. An acid that comes from an organic source, either from plants or animals such as ethanoic acid in
vinegar, citric acid in lemon and methanoic acid from ants.
2. Consists of elements : carbon, C ; hydrogen, H ; and oxygen, O.
3. The difference compared to alcohol is that carboxylic acids have two oxygen atoms whereas
alcohols have only one oxygen atom.
STARCH
GLUCOSE
. ETHENE PETROLEUM
Combustion
in excess
oxygen
Oxidation
by an oxidising
agent
Dehydration
by a dehydrating
agent
. and
.
Fermentation
Hydration
. and
.
. and
.
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
34
4. Functional group is the carboxyl group :
5. The general formula of the carboxylic acid is :
C
n
H
2n+1
COOH n = 0,1, 2, 3........
6. For straight chain carboxylic acid molecules, the name ends with oic and acid.
Activity 17 :-
Complete the following table :
Number of
carbon atoms
Molecular Formula
C
n
H
2n+1
COOH
n Structural Formula Name
1
HCOOH 0
methanoic acid
2
CH
3
COOH 1
ethanoic acid
3 2
4
5
C
O
OH
C
O
OH
H
C
O
OH
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
35
6
.
7. For branched chain carboxylic acid molecules :
- the name and position of the branched group is written as prefix
- the smallest number is given to the carbon atom that is joined to the functional carboxyl
group.
Example :
H H H O
| | | //
H C C C C
| | | \
H H CH
3
OH
2-methylbutanoic acid
8. Preparation of ethanoic acid :
Laboratory preparation through oxidation of ethanol by acidified KMnO
4
/ K
2
Cr
2
O
7
solution
H H H O
| | | //
H - C - C - OH + 2[O] H - C - C + H
2
O
| | | \
H H H OH
ethanol ethanoic acid
Activity 18 :-
Draw the set-up of apparatus for the preparation of ethanoic acid by reflux method.
(preparation of ethanoic acid in laboratory)
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
36
Industrial preparation using petroleum and natural gas.
9. Chemical properties of carboxylic acid
(i) Reacts as an acid with base, metal carbonates and metals that are more electropositive
than hydrogen.
(a) Carboxylic acid + electropositive metal carboxylate salt + hydrogen gas
(b) Carboxylic acid + base carboxylate salt + water
(c) Carboxylic acid + metal carbonate carboxylate salt + water + carbon dioxide
Activity 19 :-
Complete the following equations :
(a) HCOOH + Mg +
.. acid magnesium + ........................
(b) C
2
H
5
COOH + KOH + H
2
O
. .. + water
(c) CH
3
COOH + Na
2
CO
3
CH
3
COONa + H
2
O + CO
2
. acid sodium carbonate Sodium ethanoate + water + carbon dioxide
(d) C
2
H
3
COOH + K
2
CO
3
+ + CO
2
(e) C
2
H
5
COOH + NaOH +
(f) C
5
H
11
COOH + K
(ii) React with alcohol to produce ester and water.
Carboxylic acid reacts with alcohol to produce ester and water with the presence of
concentrated sulphuric acid as a catalyst (Esterification reaction).
Carboxylic acid + Alcohol Ester + Water
C
n
H
2n+1
COOH C
n
H
2n+1
OH C
n
H
2n+1
COOC
n
H
2n+1
H
2
O
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
37
(a) C
2
H
5
COOH + C
2
H
5
OH C
2
H
3
COOC
2
H
5
+ H
2
O
propanoic acid ethanol ethyl propanoate
(b) C
3
H
7
COOH + C
3
H
7
OH C
2
H
3
COOC
2
H
5
+ H
2
O
butanoic acid propanol propyl butanoate
(c) HCOOH + C
2
H
5
OH C
2
H
3
COOC
2
H
5
+ H
2
O
methanoic acid ethanol ethyl methanoate
(d) ethanoic acid + ethanol Ethyl ethanoat + water
H O H H
| // | |
H - C - C + HO - C - C - H
| \ | |
H OH H H
ethanoic acid ethanol
(d) butanoic acid + pentanol + water
10. Uses of Carboxylic Acid
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
38
2.10 ESTERS
1. Esters are non-hydrocarbon organic compounds containing carbon, hydrogen and oxygen.
2. General formula of ester :
O
||
R - C - O - R or RCOOR
O
||
R C - O : Derived from carboxylic acid, name ending with oate.
R is alkyl group with general formula C
n
H
2n+1
, n = 0, 1, 2, 3,
-R : Derived from alcohol, name ending with yl.
R is alkyl group with general formula C
n
H
2n+1
; n = 1, 2, 3, .
3. Formation of esters :
Esters are produced when carboxylic acid reacts with alcohol in the presence of concentrated
sulphuric acid as a catalyst (Esterification reaction) :
O O
|| ||
R - C O-H + H-O-R R - C - O - R + H-O-H
carboxylic acid alcohol ester water
Example:
CH
3
COOH + C
3
H
7
OH CH
3
COOC
3
H
7
+ H
2
O
ethanoic acid propanol prophyl ethanoate
4. Naming of esters :
Name for esters are :
first : read from the alcohol component
followed by : the carboxylic acid component.
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
39
Example:
CH
3
COOH + C
3
H
7
OH CH
3
COOC
3
H
7
+ H
2
O
ethanoic acid propanol prophyl ethanoate
or
H O H H H H O H H H
|| ||
H C C O-H H-O C C C H H C C O C C C H + H-O-H
H H H H H H H H
ethanoic acid propanol propyl ethanoate
Activity 20 :-
Complete the following equations :
(i) H2SO4
HCOOH + C
2
H
5
OH + H
2
O
methanoic acid ethanol Ethyl methanoate water
(ii) H2SO4
C
3
H
7
OH + C
4
H
9
COOH C
4
H
9
COOC
3
H
7
+ H
2
O
Propanol pentanoic acid water
(iii) H2SO4
C
3
H
7
COOH + C
2
H
5
OH + H
2
O
Butanoic acid ethanol . water
(iv)
H H H O H H H H H
| | | || | | | | |
H C C - C - C - OH + HO - C - C - C - C - C - H +
| | | | | | | |
H H H H H H H H
Butanoic acid pentanol
(v)
H H O H H H H
| | || | | | |
H C C - C - OH + HO - C - C - C - C - H +
| | | | | | |
H H H H H H H
Propanoic acid butanol ........... water
5. Physical properties of esters :
- Ester is a neutral compound with a sweet smell / fruity smell.
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
40
- Esters have low density ; less dense than water.
- Simple esters are colourless liquid at room temperature.
- Simple esters are very volatile.
- Insoluble in water.
6. Uses of esters :
Most simple esters are found naturally in fruits and flowers. The fragrance of flowers and fruits is
due to the presence of esters.
Example:
Ester Name Naturel source
CH
3
COOC
5
H
11
Pentyl ethanoate Pineapple
C
2
H
5
COOCH
3
Methyl propanoate Apple
C
3
H
7
COOC
5
H
11
Pentyl butanoate Banana
Used in the preparation of cosmetics and perfumes.
As artificial flavour in processed of food and drinks
Used in the production of polyester ( synthetic fibers for making textiles)
Natural fats are esters which react with alkalis to produce soap.
Activity 21 :-
1 Compound J is prepared by the fermentation of glucose. Diagram 1 shows a few reactions that
involve compound J with the molecular formula C
2
H
6
O.
Fermentation
Combustion
Glucose
Compound J
C
2
H
6
O
Sebatian J
C
2
H
6
O
Gas N
Compound K
C
2
H
4
O
2
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
41
(a) Name the compound J.
(b) Write the equation for the complete combustion of compound J in air.
(c) Gas M is produced when vapour of compound J is heated strongly and continuously with
aluminium oxide.
(i) Draw the set-up of the apparatus to carried out this reaction.
(ii) Name the type of reaction occur.
..
(iii) Write the molecular formula and name the gas M.
..
(iii) If gas M is passed through bromine water, predict your observation.
..
(d) Compound K is produced when compound J is reacted with oxidising agent.
(i) Name an oxidising agent that can be use in this reaction.
..
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
42
(ii) Write the chemical equation for the reaction.
..
(iii) Draw the structural formula and name the compound K.
(iii) Compound K can react with calcium carbonate. Write the chemical equation of this
reaction..
..
(e) A mixture of compound J and compound K with concentrated sulphuric acid is heated under
reflux to form compound L.
Diagram 2 shows the wrong set-up of apparatus for heating under reflux.
(i) State two mistakes shown in the set-up in Diagram 2.
DIAGRAM 2
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
43
..
..
(ii) Name the type of reaction occur.
..
(iii) Draw the structural formula of compound L.
(iv) Name the compound L in (d) (iii).
..
(v) State two uses of compound L in our daily lives.
..
..
2 Describe how the following conversions can be carried out.
[Include the reagents used, suitable conditions and the chemical equations involved]
(a)
(b)
(c)
(d)
Glucose etene
Propan-1-ol propane
Butan-1-ol butyl propanoate
Ethanol sodium ethanoate
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
44
2.11 FATS
1. Fats and oils are esters.
Fats ; a natural ester found in animal and human tissue (solid at room temperature).
Oils ; a natural ester found in fish tissue and plants (liquid at room temperature).
Formed by esterification of glycerol (alcohol) / 1,2,3-propanetriol ( alcohol with 3
hydroxyl -OH) with fatty acid (organic acid with long carbon chain, C
n
H
2n+1
COOH , n is
about 10 to 20).
(i) Esterification reaction between glycerol and fatty acid :
... + fatty acid oil or fat + water
H O H O
H C O H H O C R H C O C R
O O
H C O H + H O C R H C O C R + 3H
2
O
O O
H C O H H O C R H C O C R
H H
(ii) R, R' and R" represent hydrocarbon chains (alkyl groups) that are the same or different.
(iii) Fats are triesters (triglyceride).
2. The importance of oils and fats :
Fats and oil provide energy for our bodies.
Build membrane cell and certain hormones.
Dissolve certain vitamins for absorption.
3. Source of fats and oils :
- Fats found in animals like cow and goat, are at room temperature. Example
of animal fats are butter, cheese and lard.
- Oils from plants are at room temperature. They are called Example
of oils are peanut oil, soya bean oil and corn oil.
4. Saturated and unsaturated fat :
Fat and oil molecules are made up of two parts i.e derived from . and
derived from ..
Saturated fats molecules are esters of saturated fatty acids. Saturated fatty acids contain
.. carbon- carbon (- C - C - ) covalent bonds.
1 mol of glycerol 3 mol of fatty acid 1 mol of fat or oil 3 mol of water
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
45
Example :
Glyceryl tristearate
H O
H C O C (CH
2
)
16
CH
3
O
H C O C (CH
2
)
16
CH
3
O
H C O C (CH
2
)
16
CH
3
H
Unsaturated fats molecules are esters of unsaturated fatty acids that contain
.. and .. covalent bonds between carbon atoms in
their hydrocarbon chain.
Example :
Glyseryl trilinolate
H O
H C O C (CH
2
)
7
CH=CH-CH
2
-CH=CH-(CH
2
)
4
-CH
3
O
H C O C (CH
2
)
7
CH=CH-CH
2
-CH=CH-(CH
2
)
4
-CH
3
O
H C O C (CH
2
)
7
CH=CH-CH
2
-CH=CH-(CH
2
)
4
-CH
3
H
If there is only one double bond in a fatty acid molecule, the fats formed are monounsaturated
fat.
If there are more than one double bonds in a fatty acid molecule, the fats formed are
polyunsaturated fats.
Example : [SPM 2008]
Derived from glycerol
Derived from stearic acid (fatty acid)
fatty acids contain single
carbon- carbon (-C-C- )
covalent bonds
Derived from glycerol
Derived from linolic acid (fatty acid)
fatty acids
contain double
carbon-carbon
(-C = C- )
covalent bonds
CH(CH
2
)
7
CH C
O
OH
CH
3
(CH
2
)
7
C
O
OH
CH
3
(CH
2
)
14
..saturated
..saturated
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
46
The fats and oils are a mixture of saturated and unsaturated fats molecules.
An oil or fat is classified as a saturated/unsaturated* if it has more saturated fat
molecules compared to unsaturated fat molecules; for example animal fats.
An oil or fat is classified as an saturated/unsaturated* if it has more unsaturated fat
molecules compared to saturated fat molecules; for example vegetable oils except coconut
oil.
5. Converting unsaturated fats to saturated fats
The double covalent bonds between carbon atoms in unsaturated fats are easily oxidized.
When this happen, the fat has turn rancid (sour).
Unsaturated fats can be converted to saturated fats by process
for example in the manufacture of margarine.
Catalyst used is nickel /platinum .
It occurred at 180
o
C.
H H H H
| | Ni/Pt | |
~ C = C ~ + H
2
~ C - C ~
180
o
C | |
H H
Unsaturated fat (liquid) Saturated fat (solid)
Sources of unsaturated fats are palm oil, soya bean oil, etc.
6. The effect of fats on health
There are two types of, LDL cholesterol and cholesterol HDL cholesterol :
Double bond
Single bond
CH(CH
2
)
7
CH C
O
OH
CH
3
(CH
2
)
4
CH
2
CH CH
..saturated
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
47
LDL cholesterol; causes plaque deposits on the walls of veins or arteries which will lead
to heart attack and stroke.
HDL cholesterol; reduces deposit on the artery walls.
Animals fats (saturated fats) contain higher LDL cholesterol whereas vegetable oils (unsaturated
fats) contain higher HDL cholesterol.
Eating food high in animal fats increases the level of LDL in blood.
7. Palm Oil
Palm oil is extracted from fresh oil palm fruits. The industrial extraction of palm oil involves :
Palm oil contains 49 % of saturated fats and 51% of unsaturated fats.
It is classified as .. fats.
Palm oil is used in cooking and manufacture of margarine, soap.
2.12 NATURAL RUBBER
a. Natural rubber is a natural polymer.
Natural polymers are polymers that exist in nature and are not man made.
Example :
Natural polymer Monomer
Protein Amino acid
Carbohydrate Glucose
Natural rubber Isoprene
b. The Structure of Rubber
i. Rubber is formed from the monomer isoprene. Molecular formula of isoprene is C
5
H
8
ii. Isoprene molecules are joined together by addition .. process to form the
polymer of natural rubber, polyisoprene :
. H H CH
3
H H H CH
3
H
C = C C = C C - C = C - C
H H H H
Isoprene (2-methylbut-1,3-diene) Polyisoprene
c. Coagulation of latex
n is a large number
n
n
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
48
i. Latex is milk like liquid obtained from tapped rubber tree. Latex is a which
contains suspension of rubber particles in water.
ii. The rubber particles are made up of long chain rubber polymers [(C
5
H
8
)
n
] surrounded by
a .. The protein membrane is charged. The forces of
repulsion between negatively charged particles prevent them from combining or
coagulate.
iii. Latex coagulates when :
Acid is added to it such as methanoic acid (formic acid), ethanoic acid (acetic acid) or
any other weak acids.
or
Left aside for 1 - 2 days due to bacterial action on latex. Bacteria produces acid that
contains hydrogen ions (H
+
) which causes coagulation of latex.
Coagulate latex is semi solid.
iv. When acid is added to latex, coagulation of latex occurs :
Positively charged hydrogen ions from the acid neutralises the negative charges on the
surface of the protein membrane. A neutral rubber particle is formed.
The neutral particles no longer repel each other. These neutral particles collide with each
other, causing the membrane to break. The rubber polymers are freed and they coagulate
by combining together to form large lump of rubber polymer (solidified). The latex has
coagulated.
v. Coagulation of latex can be prevented by adding alkali (ammonia) to it. The ammonia
solution (containing OH
-
ions) will neutralise any acids that may be produced by the
bacteria.
Activity 22 :-
Protein membrane
Rubber molecule
Negative charge
Repulsion force
Rubber polymer
chain
Positive charge
carried by H
+
Membrane
break
Rubber polymers
are coagulate
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
49
Describe how the presence of an alkali can prevent the coagulation process of latex.
.
.
.
.
.
d. Properties and Uses of Natural Rubber
Activity 23 :-
Complete the following table :
Property Description Uses
Elastic
When it is stretched, it straighten out.
It return back to its original shape once the
stretching force is released.
Rubber tube, gloves,
rubber bands, shoe
soles, natural latex
modified concrete and
natural rubberised
bitumen for surfacing
roads.
Not resistance
to oxidation /
Easily oxidised
The natural rubber polymers are easily
oxidised due to presence of double bonds.
Effect of heat
When it is heated, it is soften and become
sticky.
When it is cooled, it becomes hard and brittle.
Effect of
solvent
Natural rubber is soluble in organic solvents,
alkaline and acidic solutions.
The properties of natural rubber can be improved through the vulcanisation process.
e. Vulcanisation of rubber :
i. Natural rubber is elastic.
(return to its original shape after stretching force released).
ii. When the rubber is over stretched, the rubber molecules do not return to its original
position. The rubber has lost elasticity.
iii. Natural rubber becomes more stronger and elastic after vulcanisation.
VULCANISATION
Sulphur is heated together
with natural rubber.
Rubber stripe is soaked in
sulphur monochloride solution in
methylbenzene for a few hours
and then dried.
In industry In school laboratory
4541 CHEMISTRY Chapter 11
Chapter 11 Carbon Compounds
50
Activity 24 :-
Compare the properties of natural rubber and vulcanised rubber.
Natural rubber Vulcanised rubber
In vulcanised rubber :
The sulphur atoms form cross link between the long rubber molecules.
This reduces the ability of the polymers to slide over each other.
The rubber molecules return back to its original positions after being stretched.
END OF CHAPTER 11
S
S
S
S
S
S
S
S
S
S
Cross
link
S
S
S
S
S
S
S
S
S
S
S-ar putea să vă placă și
- Introduction To Orgnic ChemistryDocument27 paginiIntroduction To Orgnic ChemistryladybugÎncă nu există evaluări
- Organic Chemistry Notes-G10Document41 paginiOrganic Chemistry Notes-G10rana alweshah100% (1)
- Chemistry SME Notes (Organic Chemmistry)Document14 paginiChemistry SME Notes (Organic Chemmistry)Sayeef MahdiÎncă nu există evaluări
- Acid-Base Practice Problems-Answers PDFDocument5 paginiAcid-Base Practice Problems-Answers PDFSuci PrameswariÎncă nu există evaluări
- Redox RxnsDocument30 paginiRedox RxnsJolaine ValloÎncă nu există evaluări
- Carboxylic Acids and EsterDocument9 paginiCarboxylic Acids and EsterNeen NaazÎncă nu există evaluări
- Edexcel & Cambridge Syllabus: Unit 4: Carbonyl Compounds Alauddin Sir A & O Level Chemistry TeacherDocument8 paginiEdexcel & Cambridge Syllabus: Unit 4: Carbonyl Compounds Alauddin Sir A & O Level Chemistry TeacherMaliha Ishrat JarinÎncă nu există evaluări
- Rate of Reaction NotesDocument27 paginiRate of Reaction NotesYong SiewkuanÎncă nu există evaluări
- Chapter 06 Phase Equilibria 4 PDF FreeDocument77 paginiChapter 06 Phase Equilibria 4 PDF FreeGabriel SilvaÎncă nu există evaluări
- Electron Transfer Reactions of Complex Ions in SolutionDe la EverandElectron Transfer Reactions of Complex Ions in SolutionÎncă nu există evaluări
- Functional Group NamesDocument21 paginiFunctional Group NamesAdine RaissaÎncă nu există evaluări
- Section A: Mcqs Halogen DerivativesDocument11 paginiSection A: Mcqs Halogen DerivativesBint A. Qadir100% (1)
- Hydroxy CompoundsDocument9 paginiHydroxy Compoundschong56Încă nu există evaluări
- 2015 H2 Carbonyl Cpds Tutorial Answer Updated PDFDocument25 pagini2015 H2 Carbonyl Cpds Tutorial Answer Updated PDFJohnÎncă nu există evaluări
- XI Chemistry Chapterwise Advanced Study MaterialDocument537 paginiXI Chemistry Chapterwise Advanced Study MaterialregisÎncă nu există evaluări
- Chemical Kinetics Part - IDocument43 paginiChemical Kinetics Part - ISanskar BhattacharyaÎncă nu există evaluări
- WTF Bharat Panchal FinalDocument135 paginiWTF Bharat Panchal FinalDhruv KarheÎncă nu există evaluări
- Back TitrateDocument16 paginiBack Titratepicket1019Încă nu există evaluări
- Chapter 22 - Alkanes and AlkenesDocument6 paginiChapter 22 - Alkanes and AlkenesJERVINLIM100% (1)
- 12 Chemistry Ncert Ch09 Coordination Compounds Part 01 QuesDocument43 pagini12 Chemistry Ncert Ch09 Coordination Compounds Part 01 Queshumayun khalidÎncă nu există evaluări
- Carboxylic AcidDocument21 paginiCarboxylic AcidShalsabila NHÎncă nu există evaluări
- Orca Share Media1573645518496Document22 paginiOrca Share Media1573645518496KhiaÎncă nu există evaluări
- 5 Key Factors That Affect Acidity in Organic Chemistry - Master Organic ChemistryDocument23 pagini5 Key Factors That Affect Acidity in Organic Chemistry - Master Organic ChemistryAhmad NisarÎncă nu există evaluări
- Hydrocarbons: K. Atkins IB Chemistry Pensacola High SchoolDocument31 paginiHydrocarbons: K. Atkins IB Chemistry Pensacola High Schoollianchen251110Încă nu există evaluări
- Chapter - 3.3 Precipitation TitrationDocument25 paginiChapter - 3.3 Precipitation TitrationDessu AshagrieÎncă nu există evaluări
- Ib PPT 10 HL PDFDocument38 paginiIb PPT 10 HL PDFzarna nirmal rawalÎncă nu există evaluări
- Experiment I1 Preparation of Some Cobaltammine ComplexesDocument7 paginiExperiment I1 Preparation of Some Cobaltammine ComplexesIftitah HauriyahÎncă nu există evaluări
- 2023-24 Coordination CompoundsDocument36 pagini2023-24 Coordination Compoundsthe Skulptor100% (1)
- Organic ChemistryDocument26 paginiOrganic Chemistryapi-379837460% (5)
- AQA As ChemistryDocument11 paginiAQA As ChemistryIlijah CorbinÎncă nu există evaluări
- H2 Atomic Structure, Stiochiometry QuestionsDocument8 paginiH2 Atomic Structure, Stiochiometry QuestionskitoniumÎncă nu există evaluări
- Difficult Questions On Organic ChemistryDocument5 paginiDifficult Questions On Organic Chemistrytarunbirbanga100% (1)
- Chapter 01 Properties of SolutionDocument70 paginiChapter 01 Properties of SolutionSharveen Gopal100% (1)
- Chapter 3 - CALCULATIONS WITH CHEMICAL FORMULASDocument24 paginiChapter 3 - CALCULATIONS WITH CHEMICAL FORMULASSai RaghavaÎncă nu există evaluări
- Volumetric Analysis: Grade XIIDocument58 paginiVolumetric Analysis: Grade XIIAS gamingÎncă nu există evaluări
- NCERT Notes For Class 12 Chemistry Chapter 2: Solutions: Solute and SolventDocument11 paginiNCERT Notes For Class 12 Chemistry Chapter 2: Solutions: Solute and Solventshradha bittuÎncă nu există evaluări
- ElectrolysisDocument25 paginiElectrolysisMuhammad UmerÎncă nu există evaluări
- Experiment 1: Preparation of 2-Iodobenzoic Acid From Anthranilic Acid (2-Amino Benzoic Acid)Document11 paginiExperiment 1: Preparation of 2-Iodobenzoic Acid From Anthranilic Acid (2-Amino Benzoic Acid)Sanjida Khandoker 1911009049Încă nu există evaluări
- Qualitative Analysis of UnknownDocument10 paginiQualitative Analysis of UnknownJulie Edington100% (1)
- F325 Transition ElementsDocument18 paginiF325 Transition ElementsDoc_CrocÎncă nu există evaluări
- Intermolecular Forces - Chemistry PracticeDocument1 paginăIntermolecular Forces - Chemistry Practicewjahx8eloo ly100% (1)
- Intro To Organic Reactions CHM457Document73 paginiIntro To Organic Reactions CHM457Zafrel ZaffÎncă nu există evaluări
- Theory of Chemical BondingDocument36 paginiTheory of Chemical BondingI Putu Adi Surya MahardikaÎncă nu există evaluări
- Chem G-9 Lesson 7 IGCSE Qs - Rates of ReactionDocument24 paginiChem G-9 Lesson 7 IGCSE Qs - Rates of ReactionKarim WaelÎncă nu există evaluări
- U3 Oxidation and Reduction PPT WatermarkDocument45 paginiU3 Oxidation and Reduction PPT Watermarkapi-125934329Încă nu există evaluări
- G R Reduction AlkaneDocument43 paginiG R Reduction AlkaneManthan HaritashÎncă nu există evaluări
- CHM 191 Introductory Practical Chemistry I - 1Document144 paginiCHM 191 Introductory Practical Chemistry I - 1Anonymous tzZcxLMeUwÎncă nu există evaluări
- Redox Electrochem H2 QuestionsDocument7 paginiRedox Electrochem H2 QuestionskitoniumÎncă nu există evaluări
- 3 Oxidation and ReductionDocument25 pagini3 Oxidation and ReductiondonutÎncă nu există evaluări
- Quiz - Coordination Compounds PDFDocument2 paginiQuiz - Coordination Compounds PDFAman JaiswalÎncă nu există evaluări
- Witting Reaction by Suman BalyaniDocument22 paginiWitting Reaction by Suman BalyaniSuman Balyani50% (2)
- Organic Chemistry WorksheetDocument3 paginiOrganic Chemistry WorksheetOrane CassanovaÎncă nu există evaluări
- PolimerDocument22 paginiPolimerDhea Kana ZhafiraÎncă nu există evaluări
- Pdf-Haloalkanes and HaloarenesDocument159 paginiPdf-Haloalkanes and HaloarenesOmkar Singh Shekhawat100% (2)
- Aromaticity NotesDocument10 paginiAromaticity NotesVirendra Singh Rajput100% (1)
- Chemistry of Reactive Intermediate FinalDocument38 paginiChemistry of Reactive Intermediate FinalTefera100% (1)
- IsomerismDocument62 paginiIsomerismsubesinghÎncă nu există evaluări
- Tetrachlorides and Oxides of Group 14 ElementsDocument9 paginiTetrachlorides and Oxides of Group 14 ElementsXue Yi LamÎncă nu există evaluări
- Enzymes: A Practical Introduction to Structure, Mechanism, and Data AnalysisDe la EverandEnzymes: A Practical Introduction to Structure, Mechanism, and Data AnalysisEvaluare: 4 din 5 stele4/5 (2)
- 5 6206254311387693082 PDFDocument1 pagină5 6206254311387693082 PDFvaogerÎncă nu există evaluări
- Transition Chapter 3Document7 paginiTransition Chapter 3vaogerÎncă nu există evaluări
- SMK Chung Hua (CF) Miri Transition Mathematics Peperiksaan Kemahiran Berfikir Aras Tinggi (Kbat) SET 2 (NOVEMBER 2015)Document2 paginiSMK Chung Hua (CF) Miri Transition Mathematics Peperiksaan Kemahiran Berfikir Aras Tinggi (Kbat) SET 2 (NOVEMBER 2015)vaogerÎncă nu există evaluări
- SC Exercise1 Chapter2 Human Nervous SystemDocument4 paginiSC Exercise1 Chapter2 Human Nervous SystemvaogerÎncă nu există evaluări
- Chapter 1 - Introduction To Chemistry TryoutDocument5 paginiChapter 1 - Introduction To Chemistry TryoutvaogerÎncă nu există evaluări
- Chapter 13 - ThermochemistryDocument22 paginiChapter 13 - ThermochemistryvaogerÎncă nu există evaluări
- Electrolysis TCH RDocument8 paginiElectrolysis TCH RvaogerÎncă nu există evaluări
- Guidelines For Preparing Proposal STF3012 - 230108Document10 paginiGuidelines For Preparing Proposal STF3012 - 230108vaogerÎncă nu există evaluări
- Manuale D LinkDocument40 paginiManuale D LinkMarco Kripto ValliÎncă nu există evaluări
- TOR ShrimpDocument7 paginiTOR ShrimpvaogerÎncă nu există evaluări
- QuizDocument1 paginăQuizvaogerÎncă nu există evaluări
- PetroSkills 2011 Training GuideDocument84 paginiPetroSkills 2011 Training Guidelogos123Încă nu există evaluări
- Generation Transmission and DistributionDocument137 paginiGeneration Transmission and Distributionsathya2040Încă nu există evaluări
- Circuit ThryDocument34 paginiCircuit ThryMathi YuvarajanÎncă nu există evaluări
- Anshu Welding PDFDocument45 paginiAnshu Welding PDFAnshu SourabhÎncă nu există evaluări
- Design and Implementation of Well TestingDocument33 paginiDesign and Implementation of Well TestingMuhammad Tahir100% (1)
- Vehicular Emissions TrinidadDocument4 paginiVehicular Emissions Trinidadstephen_debique9455Încă nu există evaluări
- Helios Uk ElsDocument22 paginiHelios Uk ElssanitermÎncă nu există evaluări
- Solution # 2: Department of Physics IIT Kanpur, Semester II, 2022-23Document4 paginiSolution # 2: Department of Physics IIT Kanpur, Semester II, 2022-23darshan sethiaÎncă nu există evaluări
- FPFFDocument77 paginiFPFF3D NFTÎncă nu există evaluări
- Overfill - Valve - 442B Size 4Document3 paginiOverfill - Valve - 442B Size 4j8164322Încă nu există evaluări
- Fault MVA Calc - PPT (Compatibility MDocument20 paginiFault MVA Calc - PPT (Compatibility MK.T.100% (1)
- 221 1 Engineering Work Suwpport by Excel Based ProgramDocument1 pagină221 1 Engineering Work Suwpport by Excel Based ProgramZoebairÎncă nu există evaluări
- Catalog 12 Section 2 TabDocument40 paginiCatalog 12 Section 2 TabAnonymous oPInocXÎncă nu există evaluări
- SFA RM 17 - 5 - Son 1Document68 paginiSFA RM 17 - 5 - Son 1Антон МандрикÎncă nu există evaluări
- Service Bulletin - ProductDocument6 paginiService Bulletin - Productraymond rizaÎncă nu există evaluări
- IC EnginesDocument17 paginiIC EnginesJames ContiÎncă nu există evaluări
- Chevron CorporationDocument32 paginiChevron CorporationEdmond Dantès100% (1)
- Pumps Rotary Gear Pump Selection GuideDocument1 paginăPumps Rotary Gear Pump Selection GuidewidhisaputrawijayaÎncă nu există evaluări
- L1 ML Waves IDocument67 paginiL1 ML Waves ISadiq QocayevÎncă nu există evaluări
- CAT 3126 Parts Manuals PDFDocument1.040 paginiCAT 3126 Parts Manuals PDFDavid B100% (5)
- Introduction To Cementingwell - 2020 PDFDocument11 paginiIntroduction To Cementingwell - 2020 PDFShijuAsÎncă nu există evaluări
- Flywheel HandoutDocument5 paginiFlywheel Handoutamanuelfitsum589Încă nu există evaluări
- GATE Chemical Engineering 2002Document10 paginiGATE Chemical Engineering 2002rahulsaini855Încă nu există evaluări
- BOOK-CRC-2001-Chemcal Properties of Material Surfaces - M. KosmulskiDocument762 paginiBOOK-CRC-2001-Chemcal Properties of Material Surfaces - M. KosmulskiQiang SunÎncă nu există evaluări
- Electrodo de Ignicion Westwood 50Document120 paginiElectrodo de Ignicion Westwood 50RichardÎncă nu există evaluări
- Data - Sheet - IG060NK37VB8 Limit SwitchDocument2 paginiData - Sheet - IG060NK37VB8 Limit SwitchJenő SerkediÎncă nu există evaluări
- Singel OutdoorDocument4 paginiSingel OutdoorMuhammad WazirÎncă nu există evaluări
- AHSP - SDA - Basis Permen 28 TH 2016Document16 paginiAHSP - SDA - Basis Permen 28 TH 2016BagusPrambudiÎncă nu există evaluări
- PS Range BrochureDocument16 paginiPS Range BrochureJorge Alberto LTÎncă nu există evaluări