Documente Academic
Documente Profesional
Documente Cultură
Control of Posture & Movement
Încărcat de
Patterson MachariaDescriere originală:
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Control of Posture & Movement
Încărcat de
Patterson MachariaDrepturi de autor:
Formate disponibile
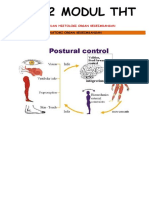
Control of Posture & Movement It is the integrated activity of multiple inputs from spinal, medullary, midbrain, and cortical
al levels that regulates the posture of the body and makes coordinated movement possible. The inputs converging on the motor neurons subserve three semidistinct functions: 1. They bring about voluntary activity; 2. They adjust body posture to provide a stable background for movement; and 3. They coordinate the action of the various muscles to make movements smooth and precise. The patterns of voluntary activity are planned within the brain, and the commands are sent to the muscles primarily via the corticospinal and corticobulbar systems. Posture is continually adjusted not only before but also during movement by postureregulating systems. Movement is smoothed and coordinated by the medial and intermediate portions of the cerebellum (spinocerebellum) and its connections. The basal ganglia and the lateral portions of the cerebellum (neocerebellum) are part of a feedback circuit to the premotor and motor cortex that is concerned with planning and organizing voluntary movement.
GENERAL PRINCIPLES Organization Motor output is of two types: reflexive, or involuntary, and voluntary. To move a limb, for example, the brain must plan a movement, arrange appropriate motion at many different joints at the same time, and adjust the motion by comparing plan with performance. The motor system "learns by doing," and performance improves with repetition. This involves synaptic plasticity. Commands for voluntary movement originate in cortical association areas. The movements are planned in the cortex as well as in the basal ganglia and the lateral portions of the cerebellar hemispheres, as indicated by increased electrical activity before the movement. The basal ganglia and cerebellum both funnel information to the premotor and motor cortex by way of the thalamus. Motor commands from the motor cortex are relayed in large part via the corticospinal tracts to the spinal cord and the corresponding corticobulbar tracts to motor neurons in the brain stem. Collaterals from these pathways and a few direct connections from the motor cortex end on brain stem nuclei, which also project to motor neurons in the brain stem and spinal cord, and these pathways, can also mediate voluntary movement. Movement sets up alterations in sensory input from the special senses and from muscles, tendons, joints, and the skin. This feedback information, which adjusts and smoothes movement, is relayed directly to the motor cortex and to the spinocerebellum. The spinocerebellum projects in turn to the brain stem. The main brain stem pathways that are concerned with posture and coordination are the rubrospinal, reticulospinal, tectospinal, and vestibulospinal tracts and corresponding projections to motor neurons in the brain stem. Control of Axial & Distal Muscles The brain stem and spinal cord, medial or ventral pathways and neurons are concerned with the control of muscles of the trunk and proximal portions of the limbs, whereas lateral pathways are concerned with the control of muscles in the distal portions of the limbs. The axial muscles are concerned with postural adjustments and gross movements, whereas the distal limb muscles are those that mediate fine, skilled movements. The ventral corticospinal tract and the medial descending paths from the brain stem (the tectospinal, reticulospinal, and vestibulospinal tracts) are concerned with adjustments of proximal muscles and posture, whereas the lateral corticospinal tract and the rubrospinal
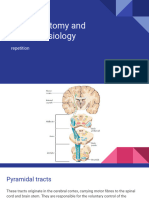
tract are concerned with distal limb muscles and, particularly in the case of the lateral corticospinal tract, with skilled voluntary movements. Phylogenetically, the medial pathways are old, whereas the lateral pathways are new. Other Terms Because the fibers of the lateral corticospinal tract form the pyramids in the medulla, the corticospinal pathways have often been referred to as the pyramidal system. The rest of the descending brain stem and spinal pathways that do not pass through the pyramids and are concerned with postural control have been called the extrapyramidal system. In addition, the motor system has often been divided into upper and lower motor neurons. Lesions of the lower motor neurons-the spinal and cranial motor neurons that directly innervate the muscles-are associated with flaccid paralysis, muscular atrophy, and absence of reflex responses. The syndrome of spastic paralysis and hyperactive stretch reflexes in the absence of muscle atrophy is said to be due to destruction of the "upper motor neurons," the neurons in the brain and spinal cord that activate the motor neurons. However, there are three types of "upper motor neurons" to consider. Lesions in many of the posture-regulating pathways cause spastic paralysis, but lesions limited to the corticospinal and corticobulbar tracts produce weakness (paresis) rather than paralysis, and the affected musculature is generally hypotonic. Cerebellar lesions produce incoordination. CORTICOSPINAL & CORTICOBULBAR SYSTEM ANATOMY & FUNCTION Tracts The nerve fibers that pass from the motor cortex to the cranial nerve nuclei form the corticobulbar tract. The nerve fibers that cross the midline in the medullary pyramids and form the lateral corticospinal tract make up about 80% of the fibers in the corticospinal pathway. The remaining 20% make up the anterior or ventral corticospinal tract which does not cross the midline until the level at which it synapses with motor neurons. In addition, this tract contains axons of corticospinal neurons that never do cross the midline and end on the same side of the body. The ventral pathway, which is the oldest phylogenetically, ends primarily on interneurons. These interneurons synapse on neurons in the medial portion of the ventral horn that control axial and proximal limb muscles. Conversely, the lateral corticospinal pathway innervates lateral neurons in the ventral horn that are concerned with distal limb muscles and hence with skilled movements. In humans, the fibers of this phylogenetically new system end directly on the lateral motor neurons. Cortical Motor Areas The cortical areas from which the corticospinal and corticobulbar system originates are generally held to be those where stimulation produces prompt discrete movement. The best known is the motor cortex (M1) in the precentral gyrus. However, there is a supplementary motor area on and above the superior bank of the cingulate sulcus on the medial side of the hemisphere that reaches to the premotor cortex on the lateral surface of the brain Motor responses are also produced by stimulation of somatic sensory area I in the postcentral gyrus and by stimulation of somatic sensory area II in the wall of the sylvian fissure.
These observations fit with the fact that 30% of the fibers making up the corticospinal and corticobulbar tracts come from the motor cortex but 30% come from the premotor cortex and 40% from the parietal lobe, especially the somatic sensory area. The various parts of the body are represented in the precentral gyrus, with the feet at the top of the gyrus and the face at the bottom. The facial area is represented bilaterally, but the rest of the representation is unilateral, the cortical motor area controlling the musculature on the opposite side of the body. The cortical representation of each body part is proportionate in size to the skill with which the part is used in fine, voluntary movement. The areas involved in speech and hand movements are especially large in the cortex; use of the pharynx, lips, and tongue to form words and of the fingers and apposable thumbs to manipulate the environment are activities in which humans are especially skilled. There is a regular representation of the body, with the axial musculature and the proximal portions of the limbs represented along the anterior edge of the precentral gyrus and the distal part of the limbs along the posterior edge. The cells in the cortical motor areas are arranged in columns. The cells in each column receive fairly extensive sensory input from the peripheral area in which they produce movement, providing the basis for feedback control of movement. Some of this input may be direct, and some is relayed from somatic sensory area I in the postcentral gyrus. Cerebral dominance, which is discussed in detail in also, affects the motor cortex in humans. Moving the fingers of the left hand is associated mainly with activation of the right motor cortex and vice versa. However, moving the fingers of the left hand also activates the left motor cortex, particularly in individuals who are right-handed. This correlates with the fact that lesions of the left motor cortex cause motor dysfunction in the left hand as well as the right hand, whereas lesions of the right motor cortex have little effect on the right hand. Plasticity In intact experimental animals and humans, the motor cortex shows the same kind of plasticity as the sensory cortex. Thus, for example, the finger areas of the contralateral motor cortex enlarge as a pattern of rapid finger movement is learned with the fingers of one hand; this change is detectable at 1 week and maximal at 4 weeks. Cortical areas of output to other muscles also increase in size when motor learning involves these muscles. When a small focal ischemic lesion is produced in the hand area of the motor cortex of monkeys, the hand area may reappear, with return of motor function, in an adjacent undamaged part of the cortex. Thus, the maps of the motor cortex are not immutable, and they change with experience. Supplementary Motor Area For the most part, the supplementary motor area projects to the motor cortex. It appears to be involved primarily in programming motor sequences. Lesions of this area in monkeys produce awkwardness in performing complex activities and difficulty with bimanual coordination. When human subjects count to themselves without speaking, the motor cortex is quiescent, but when they speak the numbers aloud as they count, blood flow increases in the motor cortex and the supplementary motor area. Thus, the supplementary motor area as well as the motor cortex is involved in voluntary movement when the movements being performed are complex and involve planning. Blood flow increases whether or not a planned movement is carried out. The increase occurs whether the movement is performed by the contralateral or the ipsilateral hand.
Premotor Cortex The premotor cortex projects to the brain stem areas concerned with postural control and to the motor cortex as well as providing part of the corticospinal and corticobulbar output. Its function is still incompletely understood, but it may be concerned with setting posture at the start of a planned movement and with getting the individual ready to perform. Posterior Parietal Cortex In addition to providing fibers that run in the corticospinal and corticobulbar tracts, the somatic sensory area and related portions of the posterior parietal lobe project to the premotor area. Lesions of the somatic sensory area cause defects in motor performance that are characterized by inability to execute learned sequences of movements such as eating with a knife and fork. Some of the neurons in area 5 are concerned with aiming the hands toward an object and manipulating it, whereas some of the neurons in area 7 are concerned with hand-eye coordination. Role in Movement The corticospinal and corticobulbar system is the primary pathway for the initiation of skilled voluntary movement. Careful section of the pyramids producing highly selective destruction of the lateral corticospinal tract in laboratory primates produces prompt and sustained loss of the ability to grasp small objects between two fingers and to make isolated movements of the wrists. However, the animal can still use the hand in a gross fashion and can stand and walk. These deficits are consistent with loss of control of the distal musculature of the limbs, which is concerned with fine skilled movements. On the other hand, lesions of the ventral corticospinal tract produce axial muscle deficits that cause difficulty with balance, walking, and climbing. Effects on Stretch Reflexes Section of the pyramids in monkeys produces prolonged hypotonia and flaccidity rather than spasticity. The anatomic arrangements in humans are such that disease processes rarely, if ever, damage the corticospinal and corticobulbar tracts without also destroying posture-regulating pathways. When spasticity is present, it is probably caused by damage to these latter pathways rather than to the corticospinal and corticobulbar tracts. Damage to the lateral corticospinal tract in humans produces the Babinski sign: dorsiflexion of the great toe and fanning of the other toes when the lateral aspect of the sole of the foot is scratched. Except in infancy, the normal response to this stimulation is plantar flexion in all the toes. The Babinski sign is believed to be a flexor withdrawal reflex that is normally held in check by the lateral corticospinal system. It is of value in the localization of disease processes, but its physiologic significance is unknown. POSTURE-REGULATING SYSTEMS Integration At the spinal cord level, afferent impulses produce simple reflex responses. At higher levels in the nervous system, neural connections of increasing complexity mediate increasingly complicated motor responses. In the intact animal, the individual motor responses are fitted into or "submerged" in the total pattern of motor activity. When the neural axis is transected, the activities integrated below the section are cut off or released from the "control of higher brain centers" and often appear to be accentuated. Release of this type, long a cardinal principle in neurology, may be due in some situations to removal of an inhibitory control by higher neural centers.
A more important cause of the apparent hyperactivity is loss of differentiation of the reaction, so that it no longer fits into the broader pattern of motor activity. An additional factor may be denervation hypersensitivity of the centers below the transection, but the role of this component remains to be determined.
Postural Control It is impossible to separate postural adjustments from voluntary movement in any rigid way, but it is possible to differentiate a series of postural reflexes that not only maintain the body in an upright, balanced position but also provide the constant adjustments necessary to maintain a stable postural background for voluntary activity. These adjustments include maintained static reflexes and dynamic, short-term phasic reflexes. The former involve sustained contraction of the musculature, whereas the latter involve transient movements. Both are integrated at various levels in the CNS from the spinal cord to the cerebral cortex and are effected largely through various motor pathways. A major factor in postural control is variation in the threshold of the spinal stretch reflexes, which is caused in turn by changes in the excitability of motor neurons and, indirectly, by changes in the rate of discharge in the efferent neurons to muscle spindles.
SPINAL INTEGRATION Spinal Shock In all vertebrates, transection of the spinal cord is followed by a period of spinal shock during which all spinal reflex responses are profoundly depressed. During this period the resting membrane potential of the spinal motor neurons is 2-6 mV greater than normal. Subsequently, reflex responses return and become relatively hyperactive. The duration of spinal shock is proportionate to the degree of encephalization of motor function in the various species. In frogs and rats it lasts for minutes; in dogs and cats it lasts for 1-2 hours; in monkeys it lasts for days; and in humans it usually lasts for a minimum of 2 weeks. The cause of spinal shock is uncertain. Cessation of tonic bombardment of spinal neurons by excitatory impulses in descending pathways undoubtedly plays a role, but the subsequent return of reflexes and their eventual hyperactivity also have to be explained. The recovery of reflex excitability may be due to the development of denervation hypersensitivity to the mediators released by the remaining spinal excitatory endings. Another possibility for which there is some evidence is the sprouting of collaterals from existing neurons, with the formation of additional excitatory endings on interneurons and motor neurons. The first reflex response to appear as spinal shock wears off in humans is frequently a slight contraction of the leg flexors and adductors in response to a noxious stimulus. In some patients, the knee jerks come back first. The interval between cord transection and the beginning return of reflex activity is about 2 weeks in the absence of any complications, but if complications are present it is much longer. It is not known why infection, malnutrition, and other complications of cord transection inhibit spinal reflex activity. Complications of Cord Transection Like all immobilized patients, they develop a negative nitrogen balance and catabolize large amounts of body protein. The weight of the body compresses the circulation to the skin over bony prominences, so that unless the patient is moved frequently the skin breaks down at these points and decubitus ulcers form.
The ulcers heal poorly and are prone to infection because of body protein depletion. The tissues that are broken down include the protein matrix of bone, and this plus the immobilization cause Ca2+ to be released in large amounts. This leads to hypercalcemia and hypercalciuria, and calcium stones often form in the urinary tract. The stones and the paralysis of bladder function both cause urinary stasis, which predisposes to urinary tract infection, the most common complication of spinal cord injury. Responses in Chronic Spinal Animals & Humans Once the spinal reflexes begin to reappear after spinal shock, their threshold steadily drops. In chronically quadriplegic humans, the threshold of the withdrawal reflex is especially low. Even minor noxious stimuli may cause not only prolonged withdrawal of one extremity but marked flexion-extension patterns in the other three limbs. Repeated flexion movements may occur for prolonged periods, and contractures of the flexor muscles develop. Stretch reflexes are also hyperactive, as are more complex reactions based on this reflex. For example, if a finger is placed on the sole of the foot of an animal after the spinal cord has been transected (spinal animal), the limb usually extends, following the finger as it is withdrawn. This magnet reaction (positive supporting reaction) involves proprioceptive as well as tactile afferents and transforms the limb into a rigid pillar to resist gravity and support the animal. Its disappearance is also in part an active phenomenon (negative supporting reaction) initiated by stretch of the extensor muscles. If the cord section is incomplete, the flexor spasms initiated by noxious stimuli can be associated with bursts of pain that are particularly bothersome. Locomotion Generator Not only can spinal cats and dogs be made to stand, but circuits intrinsic to the spinal cord produce walking movements when stimulated in a suitable fashion. There are two pattern generators for locomotion in the spinal cord, one in the cervical and one in the lumbar region. However, this does not mean that spinal animals or humans can walk without stimulation; the pattern generator has to be turned on by tonic discharge of a discrete area in the midbrain, the mesencephalic locomotor region, and of course this is only possible in patients with incomplete spinal cord transection. The generators can also be turned on in experimental animals by administration of the norepinephrine precursor L-dopa (levodopa) or the -adrenergic receptor agonist clonidine after complete section of the spinal cord. Autonomic Reflexes Reflex contractions of the full bladder and rectum occur in spinal animals and humans, although the bladder is rarely emptied completely. Hyperactive bladder reflexes can keep the bladder in a shrunken state long enough for hypertrophy and fibrosis of its wall to occur. Blood pressure is generally normal at rest, but the precise feedback regulation normally supplied by the baroreceptor reflexes is absent and wide swings in pressure are common. Bouts of sweating and blanching of the skin also occur. Sexual Reflexes Other reflex responses are present in the spinal animal, but in general they are only fragments of patterns that are integrated in the normal animal into purposeful sequences. The sexual reflexes are an example. Coordinated sexual activity depends upon a series of reflexes integrated at many neural levels and is absent after cord transection.
However, genital manipulation in male spinal animals and humans produces erection and even ejaculation. In female spinal dogs, vaginal stimulation causes tail deviation and movement of the pelvis into the copulatory position.
Mass Reflex In chronic spinal animals, afferent stimuli irradiate from one reflex center to another. When even a relatively minor noxious stimulus is applied to the skin, it may irradiate to autonomic centers and produce evacuation of the bladder and rectum, sweating, pallor, and blood pressure swings in addition to the withdrawal response. This distressing mass reflex can sometimes be used to give paraplegic patients a degree of bladder and bowel control. They can be trained to initiate urination and defecation by stroking or pinching their thighs, thus producing an intentional mass reflex.
MEDULLARY COMPONENTS Introduction In experimental animals in which the hindbrain and spinal cord are isolated from the rest of the brain by transection of the brain stem at the superior border of the pons, the most prominent finding is marked spasticity of the body musculature. The operative procedure is called decerebration, and the resulting pattern of spasticity is called decerebrate rigidity. Decerebration produces no phenomenon akin to spinal shock, and the rigidity develops as soon as the brain stem is transected. Mechanism of Decerebrate Rigidity On analysis, decerebrate rigidity is found to be spasticity due to diffuse facilitation of stretch reflexes. The facilitation is due to two factors: 1) Increased general excitability of the motor neuron pool and 2) Increase in the rate of discharge in the efferent neurons. Supraspinal Regulation of Stretch Reflexes The brain areas that facilitate and inhibit stretch reflexes generally act by increasing or decreasing spindle sensitivity. The large facilitatory area in the brain stem reticular formation discharges spontaneously, possibly in response to afferent input like the RAS. However, the smaller brain stem area that inhibits efferent discharge does not discharge spontaneously but is driven instead by fibers from the cerebral cortex and the cerebellum. The smaller brain stem area that inhibits efferent discharge does not discharge spontaneously but is driven instead by fibers from the cerebral cortex and the cerebellum. The net effect of destruction of the cerebellum in humans is hypotonia rather than spasticity. The vestibulospinal and some related descending pathways are also facilitatory to stretch reflexes and promote rigidity. Significance of Decerebrate Rigidity "A caricature of the normal standing position." What has been uncovered by decerebration, then, are the tonic, static postural reflex mechanisms that support the animal against gravity. The more common pattern of extensor rigidity in the legs and moderate flexion in the arms is actually decorticate rigidity due to lesions of the cerebral cortex, with most of the brain stem intact Tonic Labyrinthine Reflexes a. In the decerebrate animal, the pattern of rigidity in the limbs varies with the position. No righting responses are present, and the animal stays in the position in which it is put.
b. If the animal is placed on its back, the extension of all four limbs is maximal. As the animal is turned to side, the rigidity decreases, and when it is prone, the rigidity is minimal though still present. c. These changes in rigidity, the tonic labyrinthine reflexes, are initiated by the action of gravity on the otolithic organs and are effected via the vestibulospinal tracts. Tonic Neck Reflexes If the head of a decerebrate animal is moved relative to the body, changes in the pattern of rigidity occur. If the head is turned to one side, the limbs on that side ("jaw limbs") become more rigidly extended while the contralateral limbs become less so. This is the position often assumed by a normal animal looking to one side. Flexion of the head causes flexion of the forelimbs and continued extension of the hind limbs, the posture of an animal looking into a hole in the ground. Extension of the head causes flexion of the hind limbs and extension of the forelimbs, the posture of an animal looking over an obstacle. These responses are the tonic neck reflexes. They are initiated by stretch of the proprioceptors in the upper part of the neck, and they can be sustained for long periods. MIDBRAIN COMPONENTS Introduction After section of the neural axis at the superior border of the midbrain (midbrain animal), extensor rigidity like that seen in the decerebrate animal is present only when the animal lies quietly on its back. In the decerebrate animal, the rigidity, which is a static postural reflex, is prominent because there are no modifying phasic postural reflexes. Chronic midbrain animals can rise to the standing position, walk, and right themselves. While the animals are engaged in these phasic activities, the static phenomenon of rigidity is not seen. Righting Reflexes Righting reflexes operate to maintain the normal standing position and keep an animal's head upright. These reflexes are a series of responses integrated for the most part in the nuclei of the midbrain. CORTICAL COMPONENTS Effects of Decortication Removal of the cerebral cortex (decortication) produces little motor deficit in many species of mammals.
BASAL GANGLIA
Anatomic Considerations The term basal ganglia is generally applied to five structures on each side of the brain: 1. Caudate nucleus, 2. Putamen, and 3. Globus pallidus, three large nuclear masses underlying the cortical mantle, and the functionally related 4. Subthalamic nucleus (body of Luys) and 5. Substantia nigra. The globus pallidus is divided into an external and an internal segment. The substantia nigra is divided into a pars compacta and a pars reticulata. Parts of the thalamus are intimately related to the basal ganglia. The caudate nucleus and the putamen are frequently called the striatum; the putamen and the globus pallidus are sometimes called the lenticular nucleus
The main afferent connections to the basal ganglia terminate in the striatum. They include the corticostriate projection from all parts of the cerebral cortex. There is also a projection from the centromedian nucleus of the thalamus to the striatum. The principal output from the basal ganglia is from the internal segment of the globus pallidus via the thalamic fasciculus to the ventral lateral, ventral anterior, and centromedian nuclei of the thalamus. Disorders of the basal ganglia (extrapyramidal disorders) do not cause weakness or reflex changes. Their hallmark is involuntary movement (dyskinesia), causing increased movement (hyperkinesia) or decreased movement (hypokinesia) and changes in muscle tone and posture.
CEREBELLUM
Anatomic Divisions The cerebellum sits astride the main sensory and motor systems in the brain stem. It is connected to the brain stem on each side by a superior peduncle (brachium conjunctivum), middle peduncle (brachium pontis), and inferior peduncle (restiform body). The medial vermis and lateral cerebellar hemispheres are more extensively folded and fissured than the cerebral cortex; the cerebellum weighs only 10% as much as the cerebral cortex, but its surface area is about 75% of that of the cerebral cortex. Anatomically, the cerebellum is divided into three parts by two transverse fissures. The posterolateral fissure separates the medial nodulus and the lateral flocculus on either side from the rest of the cerebellum, and the primary fissure divides the remainder into an anterior and a posterior lobe. Lesser fissures divide the vermis into smaller sections, so that it contains ten primary lobules numbered I-X from superior to inferior. Functional Divisions From a functional point of view, the cerebellum is also divided into three parts, but in a different way. The nodulus in the vermis and the flanking flocculus in the hemisphere on each side form the flocculonodular lobe or vestibulocerebellum. This lobe, which is phylogenetically the oldest part of the cerebellum, has vestibular connections and is concerned with equilibrium and learning- induced changes in the VOR. The rest of the vermis and the adjacent medial portions of the hemispheres form the spinocerebellum, the region that receives proprioceptive input from the body as well as a copy of the "motor plan" from the motor cortex. By comparing plan with performance, it smoothes and coordinates movements that are ongoing. The vermis projects to the brain stem area concerned with control of axial and proximal limb muscles, whereas the hemispheres project the brain stem areas concerned with control of distal limb muscles. The lateral portions of the cerebellar hemispheres are called the neocerebellum. They are the newest from a phylogenetic point of view, reaching their greatest development in humans. They interact with the motor cortex in planning and programming movements. The archicerebellum (vestibulocerebellum) comprises the flocculonodular lobe, helps maintain equilibrium and coordinate eye-head-neck movements, and is closely interconnected with the vestibular nuclei. The midline vermis (paleocerebellum) helps coordinate movement of the trunk and legs. Vermis lesions result in abnormalities of stance and gait. The lateral hemispheres, which make up the neocerebellum, control ballistic and finely coordinated limb movements, predominantly of the arms. It appears to be important for the initial learning of motor tasks, acquisition of cognitive information, and the efficient execution of previously learned sensorimotor responses
cerebellum also may act in concert with the frontal lobes to facilitate efficient processing and retrieval of cognitive information Flocculonodular Lobe Animals in which the flocculonodular lobe has been destroyed walk in a staggering fashion on a broad base. They tend to fall and are reluctant to move without support. Similar defects are seen in children as the earliest signs of a midline cerebellar tumor that arises from cell rests in the nodule. Early in its course, it produces damage that is generally localized to the flocculonodular lobe. Selective ablation of the flocculonodular lobe in dogs abolishes the syndrome of motion sickness, whereas extensive lesions in other parts of the cerebellum and the rest of the brain fail to affect it.
Effects on Stretch Reflexes Stimulation of the cerebellar areas that receive proprioceptive input sometimes inhibits and sometimes facilitates movements evoked by stimulation of the cerebral cortex. Lesions in folia I-VI and the paramedian areas in experimental animals cause spasticity localized to the part of the body that is represented in the part of the cerebellum destroyed. However, hypotonia is characteristic of cerebellar destruction in humans. Anatomy and Physiology of CSF Circulation and Pathways The CSF is formed within the cerebral ventricular system, mainly by the choroid plexus. Each of the four ventricles contains choroid plexus tissue, consisting of villous folds lined by epithelium and a central core of highly vascularized connective tissue. The fluid is formed by active secretion and diffusion. Nonchoroidal sources of CSF exist The net direction of CSF flow is from the lateral ventricles to the third ventricle and hence to the fourth ventricle and then through the basal cisterns, tentorium, and subarachnoid space over the cerebral convexities to the area of the sagittal sinus, from where absorption into the systemic circulation takes place. The net flow of fluid in the spinal subarachnoid space is cephalad. Most CSF absorption takes place across the arachnoid villi into the venous channels of the sagittal sinus, but fluid is also absorbed across the ependymal lining of the ventricular system and from the spinal subarachnoid space. In the normal adult, the total volume of CSF is approximately 150 mL, of which 25% is within the ventricular system. CSF is formed at the rate of approximately 20 mL/hour, thereby indicating that CSF turns over three or four times per day. CSF formation continues when the intracranial pressure rises, unless extremely high levels are reached. Thus, there must be some absorption of fluid to accommodate the volume of CSF being formed each day. Tight junctions characteristically surround the apical margins of the cells in epithelia such as the intestinal mucosa, the walls of the renal tubules, and the choroid plexus. The tight junctions between capillary endothelial cells in the brain and between the epithelial cells in the choroid plexus effectively prevent proteins from entering the brain in adults and slow the penetration of smaller molecules.
S-ar putea să vă placă și
- Gale Researcher Guide for: Overview of Physiology and NeuropsychologyDe la EverandGale Researcher Guide for: Overview of Physiology and NeuropsychologyÎncă nu există evaluări
- Motor Systems 10 05Document11 paginiMotor Systems 10 05souvik5000100% (1)
- Mind at Rest: How Neuron Structure Evolves in the Sleep Cycle.De la EverandMind at Rest: How Neuron Structure Evolves in the Sleep Cycle.Încă nu există evaluări
- Locomotor System (Text)Document3 paginiLocomotor System (Text)Aknur JakhanshaÎncă nu există evaluări
- Physio - Psych Chapter-8 - Control-Of-Movemnt - Fact-SheetDocument9 paginiPhysio - Psych Chapter-8 - Control-Of-Movemnt - Fact-SheetJustynmae JusainÎncă nu există evaluări
- Motor SystemDocument49 paginiMotor SystemAhaisibwe GordonÎncă nu există evaluări
- Cerebellum in Overall Motor ControlDocument5 paginiCerebellum in Overall Motor Controldncm.alejandroÎncă nu există evaluări
- CORTICAL & BRAINSTEM CONTROL OF MOTOR FUNCTIONSDocument268 paginiCORTICAL & BRAINSTEM CONTROL OF MOTOR FUNCTIONSsaraÎncă nu există evaluări
- Neural Control of LocomotionDocument11 paginiNeural Control of Locomotionphysiovipin86% (7)
- CerebellumDocument51 paginiCerebellumLiya ThaaraÎncă nu există evaluări
- LECTURE On Spinal CordDocument11 paginiLECTURE On Spinal CordElijah KamaniÎncă nu există evaluări
- 3motor TestingDocument74 pagini3motor Testing2013SecBÎncă nu există evaluări
- The Motor System Lecture 1 OverviewDocument7 paginiThe Motor System Lecture 1 OverviewUsman Ali AkbarÎncă nu există evaluări
- LBM 2 THTDocument20 paginiLBM 2 THTelly nurkhikmahÎncă nu există evaluări
- L1 Motor Function of CNSDocument48 paginiL1 Motor Function of CNSAhmed JawdetÎncă nu există evaluări
- Spinal ReportDocument28 paginiSpinal ReportJohngie QuilangÎncă nu există evaluări
- Cerebellum: Movement Regulation and Cognitive Functions Course Title DateDocument16 paginiCerebellum: Movement Regulation and Cognitive Functions Course Title DateDanyÎncă nu există evaluări
- Scientific Basis of Body Mapping and Cortical MapsDocument2 paginiScientific Basis of Body Mapping and Cortical MapsDssalamancazÎncă nu există evaluări
- The Cerebelum 2Document53 paginiThe Cerebelum 2Reagan MwesigwaÎncă nu există evaluări
- Cerebellum Essay - Connections and Functions of The CerebellumDocument4 paginiCerebellum Essay - Connections and Functions of The CerebellumscholifyÎncă nu există evaluări
- Physio Psy Module 8Document4 paginiPhysio Psy Module 8Kier Elizalde VistalÎncă nu există evaluări
- Neurophysiology II 2023Document90 paginiNeurophysiology II 2023trsvscpz79Încă nu există evaluări
- Neuro anatomy, motor neuronDocument9 paginiNeuro anatomy, motor neuronMichele Silva PiresÎncă nu există evaluări
- Ectodermal Derivatives of Pig EmbryoDocument7 paginiEctodermal Derivatives of Pig EmbryoChristalie Bea FernandezÎncă nu există evaluări
- ASLI - 20.10.2022NMD-5 Anatomy of Motor ControlDocument40 paginiASLI - 20.10.2022NMD-5 Anatomy of Motor ControlYorgos TheodosiadisÎncă nu există evaluări
- Neuro AnatomyDocument26 paginiNeuro Anatomymeryclave345Încă nu există evaluări
- Physiology of Motor TractsDocument34 paginiPhysiology of Motor TractsNiko MartinÎncă nu există evaluări
- The descending pathways that control motor activityDocument2 paginiThe descending pathways that control motor activityalmastar officeÎncă nu există evaluări
- Cerebellar Function TestDocument37 paginiCerebellar Function TestAdrian Paolo Malamug100% (1)
- SASDocument14 paginiSASNicole Ken AgdanaÎncă nu există evaluări
- Ch. 5Document8 paginiCh. 5Sabrina MaciarielloÎncă nu există evaluări
- Session #22 SAS - AnaPhy (Lab)Document14 paginiSession #22 SAS - AnaPhy (Lab)G IÎncă nu există evaluări
- Jingying T.A. Department of PhysiologyDocument19 paginiJingying T.A. Department of Physiologyapi-19916399Încă nu există evaluări
- PBLDocument3 paginiPBLAbi AuxÎncă nu există evaluări
- Understanding Human Anatomy and Brain FunctionDocument61 paginiUnderstanding Human Anatomy and Brain Functionjustice88_639020831Încă nu există evaluări
- Neurotransmitter Assingment Ashish Singh PDFDocument7 paginiNeurotransmitter Assingment Ashish Singh PDFAshish SinghÎncă nu există evaluări
- Motor Control IIDocument39 paginiMotor Control IILuis Eduardo RibeiroÎncă nu există evaluări
- Skeletal Motor Control HierarchyDocument16 paginiSkeletal Motor Control HierarchyHapy ArdiaÎncă nu există evaluări
- CerebellumDocument16 paginiCerebellumjainilÎncă nu există evaluări
- Systems Neuroscience Nov. 12, 2019Document37 paginiSystems Neuroscience Nov. 12, 2019TrajceÎncă nu există evaluări
- Corticospinal Tract: Functions and Clinical RelevanceDocument4 paginiCorticospinal Tract: Functions and Clinical RelevanceNrs Sani Sule MashiÎncă nu există evaluări
- Somatosensory Pathways: Peripheral Receptors and Central PathwaysDocument28 paginiSomatosensory Pathways: Peripheral Receptors and Central PathwaysJean Pierre Chastre LuzaÎncă nu există evaluări
- Neuroanatomy, Extrapyramidal System: AuthorsDocument7 paginiNeuroanatomy, Extrapyramidal System: AuthorsDiana CastilloÎncă nu există evaluări
- Motor system overview: Upper and lower motor neuronsDocument12 paginiMotor system overview: Upper and lower motor neuronssimi yÎncă nu există evaluări
- Human Nervous System - Movement - BritannicaDocument15 paginiHuman Nervous System - Movement - Britannicasalamy amourÎncă nu există evaluări
- Sensory PathwayDocument31 paginiSensory PathwayAlma BaterinaÎncă nu există evaluări
- Chapter SummaryDocument2 paginiChapter SummaryrogelynlumasagÎncă nu există evaluări
- Motor Control Theories and PhysiologyDocument7 paginiMotor Control Theories and PhysiologyAaminah NawazÎncă nu există evaluări
- Upper Motor Neuron PDFDocument3 paginiUpper Motor Neuron PDFkevin_jawanÎncă nu există evaluări
- Basic Information About NeuropsychologyDocument9 paginiBasic Information About NeuropsychologyYara Bou AbdoÎncă nu există evaluări
- 2.update Sensorik-Motorik NeuropsikiatriDocument56 pagini2.update Sensorik-Motorik NeuropsikiatriumamitÎncă nu există evaluări
- Chapter 10 Body MovementDocument27 paginiChapter 10 Body MovementHarpreet SinghÎncă nu există evaluări
- Nervous System AnswerDocument9 paginiNervous System AnswerVann VelascoÎncă nu există evaluări
- Ballon, Rosemarie E. Chapter 4Document3 paginiBallon, Rosemarie E. Chapter 4Rose Marie BallonÎncă nu există evaluări
- Motor Functions of Spinal CordDocument43 paginiMotor Functions of Spinal CordEugeniu CoretchiÎncă nu există evaluări
- Assesment NeuroDocument15 paginiAssesment Neuroerfan ghadirianÎncă nu există evaluări
- Lo 1 Week 5 NeuroDocument36 paginiLo 1 Week 5 NeuroInna DamaÎncă nu există evaluări
- Hes006 CNS Lab 15 18Document50 paginiHes006 CNS Lab 15 18Joana Elizabeth A. CastroÎncă nu există evaluări
- MBBC 2018 Notes 1Document14 paginiMBBC 2018 Notes 1Dimitri KhooÎncă nu există evaluări
- Exploring the Automatic Responses of ReflexesDocument2 paginiExploring the Automatic Responses of Reflexesmohitnet1327Încă nu există evaluări
- Mandibular FracturesDocument70 paginiMandibular FracturesPatterson MachariaÎncă nu există evaluări
- Developmental Disorders of TeethDocument64 paginiDevelopmental Disorders of TeethPatterson MachariaÎncă nu există evaluări
- Biology of Tooth MovementDocument5 paginiBiology of Tooth MovementPatterson Macharia100% (1)
- Limbic System (Behaviour and Emotion)Document19 paginiLimbic System (Behaviour and Emotion)Patterson MachariaÎncă nu există evaluări
- Development of The MaxillaDocument7 paginiDevelopment of The MaxillaShinta Syafe'iÎncă nu există evaluări
- Smell & TasteDocument7 paginiSmell & TastePatterson MachariaÎncă nu există evaluări
- Moisture ControlDocument43 paginiMoisture ControlPatterson Macharia100% (1)
- Higher Functions of The Nervous SystemDocument8 paginiHigher Functions of The Nervous SystemPatterson MachariaÎncă nu există evaluări
- Alert Behavior, Sleep and The Electrical Activity of The BrainDocument6 paginiAlert Behavior, Sleep and The Electrical Activity of The BrainPatterson MachariaÎncă nu există evaluări
- Hearing and EquilibriumDocument11 paginiHearing and EquilibriumPatterson MachariaÎncă nu există evaluări
- ReflexesDocument6 paginiReflexesPatterson MachariaÎncă nu există evaluări
- Cutaneous, Deep and Visceral SensationDocument12 paginiCutaneous, Deep and Visceral SensationPatterson MachariaÎncă nu există evaluări
- Neural Basis of Instinctual BehaviorDocument8 paginiNeural Basis of Instinctual BehaviorPatterson MachariaÎncă nu există evaluări
- CNS PharmacologyDocument26 paginiCNS PharmacologyPatterson MachariaÎncă nu există evaluări
- Adrenergic Receptor AntagonistsDocument9 paginiAdrenergic Receptor AntagonistsPatterson MachariaÎncă nu există evaluări
- Central Regulation of Visceral FunctionDocument6 paginiCentral Regulation of Visceral FunctionPatterson MachariaÎncă nu există evaluări
- Does Sastra Prescribes Animal Sacrifices For The Sake of ReligionDocument15 paginiDoes Sastra Prescribes Animal Sacrifices For The Sake of Religionkeshav3kashmiriÎncă nu există evaluări
- Antiprotozoal Agents 6Document29 paginiAntiprotozoal Agents 6EinsteenÎncă nu există evaluări
- A Detailed Lesson Plan in Partial Fulfillment of The Course Requirements in (PED8)Document11 paginiA Detailed Lesson Plan in Partial Fulfillment of The Course Requirements in (PED8)Angel Clarence BasilioÎncă nu există evaluări
- Module Possessive Adj. Lesson 3Document29 paginiModule Possessive Adj. Lesson 3Lance TuvillaÎncă nu există evaluări
- July Aug MenuDocument1 paginăJuly Aug Menusales6916Încă nu există evaluări
- Nursery RhymesDocument10 paginiNursery RhymesDegee O. GonzalesÎncă nu există evaluări
- Removable Partial DentureDocument4 paginiRemovable Partial DentureRodriguez VanesaÎncă nu există evaluări
- American Rabbit Breeders Association Guide (ARBADocument11 paginiAmerican Rabbit Breeders Association Guide (ARBANaniÎncă nu există evaluări
- Plitvice Lakes, CroatiaDocument7 paginiPlitvice Lakes, CroatiaMirkoÎncă nu există evaluări
- Catclass NotesDocument3 paginiCatclass NotesIssac CaldwellÎncă nu există evaluări
- Fluency Passages P1Document99 paginiFluency Passages P1princerafa100% (5)
- Animal Science HandoutDocument5 paginiAnimal Science Handoutromnickatchecoso14Încă nu există evaluări
- Normal Adult Laboratory ValuesDocument7 paginiNormal Adult Laboratory Valuessteinway007Încă nu există evaluări
- Organ DonationDocument3 paginiOrgan DonationGrant DonovanÎncă nu există evaluări
- Vaults of Vaarn #1 (v3) (Booklet) (2021-01-11)Document26 paginiVaults of Vaarn #1 (v3) (Booklet) (2021-01-11)Alex TrifanÎncă nu există evaluări
- ECG BasicsDocument15 paginiECG Basicsrohitmeena2889Încă nu există evaluări
- Histology AssignmentDocument4 paginiHistology AssignmentNico LokoÎncă nu există evaluări
- Y1 Module Pet ShowDocument13 paginiY1 Module Pet ShowJayne Frances EngelbertÎncă nu există evaluări
- The Little SquirrelDocument10 paginiThe Little SquirrelNgọc Ngọc100% (10)
- Ultimate Arcane SpellbookDocument32 paginiUltimate Arcane SpellbookSigraine75% (4)
- AnatomyDocument4 paginiAnatomySureen PaduaÎncă nu există evaluări
- Spiral FlapDocument20 paginiSpiral FlapDiolanda De AbreuÎncă nu există evaluări
- The Sign of The BeaverDocument13 paginiThe Sign of The Beaverjuan marcos100% (1)
- Acute Respiratory InfectionDocument32 paginiAcute Respiratory InfectionRinnaAyuNovitaSary100% (1)
- Science Notes Sem1 PDFDocument31 paginiScience Notes Sem1 PDFWolfie Saraswathi Santhosham100% (1)
- 2007 IGCSE Biology Written Paper Question PaperDocument24 pagini2007 IGCSE Biology Written Paper Question Paperseyka4Încă nu există evaluări
- Shark Finning Report WildaidDocument16 paginiShark Finning Report WildaidGlobal Wildlife Warriors100% (1)
- Eduardo Serrão - Potential Business For Chickens and Pigs and Their Products in Timor-LesteDocument6 paginiEduardo Serrão - Potential Business For Chickens and Pigs and Their Products in Timor-LestePapers and Powerpoints from UNTL-VU Joint Conferenes in DiliÎncă nu există evaluări
- A - Specialist Catalogue Fall 2019-Web PDFDocument29 paginiA - Specialist Catalogue Fall 2019-Web PDFFelipe YacumalÎncă nu există evaluări
- The Age of Magical Overthinking: Notes on Modern IrrationalityDe la EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityEvaluare: 4 din 5 stele4/5 (13)
- Techniques Exercises And Tricks For Memory ImprovementDe la EverandTechniques Exercises And Tricks For Memory ImprovementEvaluare: 4.5 din 5 stele4.5/5 (40)
- The Ultimate Guide To Memory Improvement TechniquesDe la EverandThe Ultimate Guide To Memory Improvement TechniquesEvaluare: 5 din 5 stele5/5 (34)
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeDe la EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeÎncă nu există evaluări
- The Happiness Trap: How to Stop Struggling and Start LivingDe la EverandThe Happiness Trap: How to Stop Struggling and Start LivingEvaluare: 4 din 5 stele4/5 (1)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedDe la EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedEvaluare: 5 din 5 stele5/5 (78)
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionDe la EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionEvaluare: 4 din 5 stele4/5 (402)
- Why We Die: The New Science of Aging and the Quest for ImmortalityDe la EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityEvaluare: 3.5 din 5 stele3.5/5 (2)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsDe la EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsEvaluare: 3.5 din 5 stele3.5/5 (3)
- Raising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsDe la EverandRaising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsEvaluare: 4.5 din 5 stele4.5/5 (169)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsDe la EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsEvaluare: 5 din 5 stele5/5 (1)
- The Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsDe la EverandThe Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsÎncă nu există evaluări
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaDe la EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaEvaluare: 4.5 din 5 stele4.5/5 (266)
- Mindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessDe la EverandMindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessEvaluare: 4.5 din 5 stele4.5/5 (327)
- Outlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisDe la EverandOutlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisEvaluare: 4 din 5 stele4/5 (1)
- The Tennis Partner: A Doctor's Story of Friendship and LossDe la EverandThe Tennis Partner: A Doctor's Story of Friendship and LossEvaluare: 4.5 din 5 stele4.5/5 (4)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.De la EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Evaluare: 4.5 din 5 stele4.5/5 (110)
- Cult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryDe la EverandCult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryEvaluare: 4 din 5 stele4/5 (44)
- The Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeDe la EverandThe Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeEvaluare: 4.5 din 5 stele4.5/5 (253)
- The Stress-Proof Brain: Master Your Emotional Response to Stress Using Mindfulness and NeuroplasticityDe la EverandThe Stress-Proof Brain: Master Your Emotional Response to Stress Using Mindfulness and NeuroplasticityEvaluare: 4.5 din 5 stele4.5/5 (109)
- Secure Love: Create a Relationship That Lasts a LifetimeDe la EverandSecure Love: Create a Relationship That Lasts a LifetimeEvaluare: 5 din 5 stele5/5 (17)
- Summary: It Didn't Start with You: How Inherited Family Trauma Shapes Who We Are and How to End the Cycle By Mark Wolynn: Key Takeaways, Summary & AnalysisDe la EverandSummary: It Didn't Start with You: How Inherited Family Trauma Shapes Who We Are and How to End the Cycle By Mark Wolynn: Key Takeaways, Summary & AnalysisEvaluare: 5 din 5 stele5/5 (3)
- Summary: Hidden Potential: The Science of Achieving Greater Things By Adam Grant: Key Takeaways, Summary and AnalysisDe la EverandSummary: Hidden Potential: The Science of Achieving Greater Things By Adam Grant: Key Takeaways, Summary and AnalysisEvaluare: 4.5 din 5 stele4.5/5 (15)
- Troubled: A Memoir of Foster Care, Family, and Social ClassDe la EverandTroubled: A Memoir of Foster Care, Family, and Social ClassEvaluare: 4.5 din 5 stele4.5/5 (23)
- Hearts of Darkness: Serial Killers, The Behavioral Science Unit, and My Life as a Woman in the FBIDe la EverandHearts of Darkness: Serial Killers, The Behavioral Science Unit, and My Life as a Woman in the FBIEvaluare: 4 din 5 stele4/5 (19)
- Summary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisDe la EverandSummary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisEvaluare: 5 din 5 stele5/5 (8)
- Daniel Kahneman's "Thinking Fast and Slow": A Macat AnalysisDe la EverandDaniel Kahneman's "Thinking Fast and Slow": A Macat AnalysisEvaluare: 3.5 din 5 stele3.5/5 (130)