Documente Academic
Documente Profesional
Documente Cultură
Electromagnetic Radiation
Încărcat de
Daniel EhieduDrepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Electromagnetic Radiation
Încărcat de
Daniel EhieduDrepturi de autor:
Formate disponibile
ELECTROMAGNETIC RADIATION (EMR)
By Prof. J.N.Egila Do you listen to the radio, watch TV, or use a microwave oven? All these devices make use of electromagnetic waves. Radio waves, microwaves, visible light, and x rays are all examples of electromagnetic waves that differ from each other in wavelength. Electromagnetic waves are produced by the motion of electrically charged particles. These waves are also called "electromagnetic radiation" because they radiate from the electrically charged particles. They travel through empty space as well as through air and other substances. Scientists have observed that electromagnetic radiation has a dual "personality." Besides acting like waves, it acts like a stream of particles (called "photons") that have no mass. The photons with the highest energy correspond to the shortest wavelengths Electromagnetic radiation (EMR) is energy in waves (like visible light), emitted from a source. It travels at the speed of light This energy is both electrical and magnetic. The waves alternate rapidly, from positive to negative in electrical terms, and from North to South pole in magnetic terms. Electricity and magnetism are very closely related in nature. For example, when an alternating magnetic wave penetrates a body (including yours!) an alternating electric current will flow inside that body. Electromagnetic radiation (often abbreviated E-M radiation or EMR) is a form of energy exhibiting wave-like behavior as it travels through space. EMR has both electric and magnetic field components, which oscillate in phase perpendicular to each other and perpendicular to the direction of energy propagation. Electromagnetic radiation is classified according to the frequency of its wave. The electromagnetic spectrum, in order of increasing frequency and decreasing wavelength, consists of radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays and gamma rays. The eyes of various organisms sense a small and somewhat variable window of frequencies called the visible spectrum. The photon is the quantum of the electromagnetic interaction and the basic "unit" of light and all other forms of electromagnetic radiation and is also the force carrier for the electromagnetic force. Electromagnetic radiation carries energy and momentum that may be imparted to matter with which it interacts. James Clerk Maxwell first formally postulated electromagnetic
waves. These were subsequently confirmed by Heinrich Hertz. Maxwell derived a wave form of the electric and magnetic equations, thus uncovering the wave-like nature of electric and magnetic fields, and their symmetry. Because the speed of EM waves predicted by the wave equation coincided with the measured speed of light, Maxwell concluded that light itself is an EM wave. Fig 1 ELECTROMAGNETISM
James Clerk Maxwell first formally postulated electromagnetic waves. These were subsequently confirmed by Heinrich Hertz. Maxwell derived a wave form of the electric and magnetic equations, thus uncovering the wave-like nature of electric and magnetic fields, and their symmetry. Because the speed of EM waves predicted by the wave equation coincided with the measured speed of light, Maxwell concluded that light itself is an EM wave. According to Maxwell's equations, a spatially varying electric field causes the magnetic field to change over time. Likewise, a spatially varying magnetic field causes changes over time in the electric field. In an electromagnetic wave, the changes induced by the electric field shift the wave in the magnetic field in one direction; the action of the magnetic field shifts the electric field in the same direction. Together, these fields form a propagating electromagnetic wave. A quantum theory of the interaction between electromagnetic radiation and matter such as electrons is described by the theory of quantum electrodynamics.
Fig 2
Fig 3 THE ELECTROMAGNETIC SPECTRUM
The physics of electromagnetic radiation is electrodynamics. Electromagnetism is the physical phenomenon associated with the theory of electrodynamics. Electric and
magnetic fields obey the properties of superposition. Thus, a field due to any particular particle or time-varying electric or magnetic field contributes to the fields present in the same space due to other causes. Further, as they are vector fields, all magnetic and electric field vectors add together according to vector addition. For example, in optics two or more coherent lightwaves may interact and by constructive or destructive interference yield a resultant irradiance deviating from the sum of the component irradiances of the individual lightwaves. Since light is an oscillation it is not affected by travelling through static electric or magnetic fields in a linear medium such as a vacuum. However in nonlinear media, such as some crystals, interactions can occur between light and static electric and magnetic fields these interactions include the Faraday effect and the Kerr effect. In refraction, a wave crossing from one medium to another of different density alters its speed and direction upon entering the new medium. The ratio of the refractive indices of the media determines the degree of refraction, and is summarized by Snell's law. Light of composite wavelengths (natural sunlight) disperses into a visible spectrum passing through a prism, because of the wavelength dependent refractive index of the prism material (dispersion); that is, each component wave within the composite light is bent a different amount. EM radiation exhibits both wave properties and particle properties at the same time (see wave-particle duality). Both wave and particle characteristics have been confirmed in a large number of experiments. Wave characteristics are more apparent when EM radiation is measured over relatively large timescales and over large distances while particle characteristics are more evident when measuring small timescales and distances. For example, when electromagnetic radiation is absorbed by matter, particle-like properties will be more obvious when the average number of photons in the cube of the relevant wavelength is much smaller than 1. Upon absorption of light, it is not too difficult to experimentally observe non-uniform deposition of energy. Strictly speaking, however, this alone is not evidence of "particulate" behavior of light, rather it reflects the quantum nature of matter.[1] There are experiments in which the wave and particle natures of electromagnetic waves appear in the same experiment, such as the self-interference of a single photon. True single-photon experiments (in a quantum optical sense) can be done today in undergraduate-level labs.[2] When a single photon is sent through an interferometer, it passes through both paths, interfering with itself, as waves do, yet is detected by a photomultiplier or other sensitive detector only once.
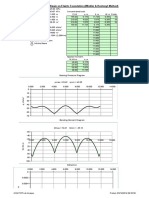
WAVE MODEL Electromagnetic radiation is a transverse wave meaning that the oscillations of the waves are perpendicular to the direction of energy transfer and travel. An important aspect of the nature of light is frequency. The frequency of a wave is its rate of oscillation and is measured in hertz, the SI unit of frequency, where one hertz is equal to one oscillation per second. Light usually has a spectrum of frequencies which sum together to form the resultant wave. Different frequencies undergo different angles of refraction. A wave consists of successive troughs and crests, and the distance between two adjacent crests or troughs is called the wavelength. Waves of the electromagnetic spectrum vary in size, from very long radio waves the size of buildings to very short gamma rays smaller than atom nuclei. Frequency is inversely proportional to wavelength, according to the equation:
where v is the speed of the wave (c in a vacuum, or less in other media), f is the frequency and is the wavelength. As waves cross boundaries between different media, their speeds change but their frequencies remain constant. Interference is the superposition of two or more waves resulting in a new wave pattern. If the fields have components in the same direction, they constructively interfere, while opposite directions cause destructive interference. The energy in electromagnetic waves is sometimes called radiant energy. Generally, EM radiation (the designation 'radiation' excludes static electric and magnetic and near fields) is classified by wavelength into radio, microwave, infrared, the visible region we perceive as light, ultraviolet, X-rays and gamma rays. Arbitrary electromagnetic waves can always be expressed by Fourier analysis in terms of sinusoidal monochromatic waves which can be classified into these regions of the spectrum. The behavior of EM radiation depends on its wavelength. Higher frequencies have shorter wavelengths, and lower frequencies have longer wavelengths. When EM radiation interacts with single atoms and molecules, its behavior depends on the amount of energy per quantum it carries. Spectroscopy can detect a much wider region of the EM spectrum than the visible range of 400 nm to 700 nm. A common laboratory spectroscope can detect wavelengths from 2 nm to 2500 nm. Detailed information about the physical properties of objects, gases, or even stars can be obtained from this type of device. It is widely used in astrophysics. For example, hydrogen atoms emit radio waves of wavelength 21.12 cm.
Soundwaves are not electromagnetic radiation. At the lower end of the electromagnetic spectrum, about 20 Hz to about 20 kHz, are frequencies that might be considered in the audio range. However, electromagnetic waves cannot be directly perceived by human ears. Sound waves are the oscillating compression of molecules. To be heard, electromagnetic radiation must be converted to air pressure waves, or if the ear is submerged, water pressure waves. LIGHT AS A WAVE FORM Waves Any wave is essentially just a way of shifting energy from one place to another - whether the fairly obvious transfer of energy in waves on the sea or in the much more difficult-toimagine waves in light. In waves on water, the energy is transferred by the movement of water molecules. But a particular water molecule doesn't travel all the way across the Atlantic - or even all the way across a pond. Depending on the depth of the water, water molecules follow a roughly circular path. As they move up to the top of the circle, the wave builds to a crest; as they move down again, you get a trough. The energy is transferred by relatively small local movements in the environment. With water waves it is fairly easy to draw diagrams to show this happening with real molecules. With light it is more difficult. The energy in light travels because of local fluctuating changes in electrical and magnetic fields - hence "electromagnetic" radiation. Wavelength, frequency and the speed of light If you draw a beam of light in the form of a wave (without worrying too much about what exactly is causing the wave!), the distance between two crests is called the wavelength of the light. (It could equally well be the distance between two troughs or any other two identical positions on the wave.)
You have to picture these wave crests as moving from left to right. If you counted the number of crests passing a particular point per second, you have the frequency of the light. It is measured in what used to be called "cycles per second", but is now called Hertz, Hz. Cycles per second and Hertz mean exactly the same thing. Orange light, for example, has a frequency of about 5 x 1014 Hz (often quoted as 5 x 108 MHz - megahertz). That means that 5 x 1014 wave peaks pass a given point every second. Light has a constant speed through a given substance. For example, it always travels at a speed of approximately 3 x 108 metres per second in a vacuum. This is actually the speed that all electromagnetic radiation travels - not just visible light. There is a simple relationship between the wavelength and frequency of a particular colour of light and the speed of light:
. . . and you can rearrange this to work out the wavelength from a given frequency and vice versa:
These relationships mean that if you increase the frequency, you must decrease the wavelength.
Compare this diagram with the similar one above.
. . . and, of course, the opposite is true. If the wavelength is longer, the frequency is lower. It is really important that you feel comfortable with the relationship between frequency and wavelength. If you are given two figures for the wavelengths of two different colours of light, you need to have an immediate feel for which one has the higher frequency. For example, if you were told that a particular colour of red light had a wavelength of 650 nm, and a green had a wavelength of 540 nm, it is important for you to know which has the higher frequency. (It's the green - a shorter wavelength means a higher frequency. Don't go on until that feels right!) Note: nm = nanometre = 10-9 metre The frequency of light and its energy Each particular frequency of light has a particular energy associated with it, given by another simple equation:
You can see that the higher the frequency, the higher the energy of the light. So . . . have you got this sorted out? Try it! Light which has wavelengths of around 380 - 435 nm is seen as a sequence of violet colours. Various red colours have wavelengths around 625 - 740 nm. Which has the highest energy? The light with the highest energy will be the one with the highest frequency - that will be the one with the smallest wavelength. In other words, violet light at the 380 nm end of its range. EM radiation with a wavelength between approximately 400 nm and 700 nm is directly detected by the human eye and perceived as visible light. Other wavelengths, especially
nearby infrared (longer than 700 nm) and ultraviolet (shorter than 400 nm) are also sometimes referred to as light, especially when visibility to humans is not relevant. If radiation having a frequency in the visible region of the EM spectrum reflects off of an object, say, a bowl of fruit, and then strikes our eyes, this results in our visual perception of the scene. Our brain's visual system processes the multitude of reflected frequencies into different shades and hues, and through this not-entirely-understood psychophysical phenomenon, most people perceive a bowl of fruit. At most wavelengths, however, the information carried by electromagnetic radiation is not directly detected by human senses. Natural sources produce EM radiation across the spectrum, and our technology can also manipulate a broad range of wavelengths. Optical fiber transmits light which, although not suitable for direct viewing, can carry data that can be translated into sound or an image. To be meaningful both transmitter and receiver must use some agreed-upon encoding system - especially so if the transmission is digital as opposed to the analog nature of the waves. COMPONENTS OF ELECTROMAGNETIC RADIATION Frequency The frequency of a wave tells us how fast the wave oscillates in cycles per second, also called Hertz (or Hz). A cycle is one oscillation of the wave (one peak and one trough). The range of all frequencies of electromagnetic radiation is collectively known as the Electromagnetic Spectrum. Wavelength The wavelength of a wave is the distance between two successive wave crests. Wavelength is inversely proportional to frequency - the higher the frequency, the shorter the wavelength. Electromagnetic wavelengths of interest to us span a huge range - from about 5000 km (long wave) to less than a billionth of a meter (gamma rays). Intensity In practical terms, the intensity or strength of an electromagnetic field at any particular point depends upon
the amount of electrical and magnetic energy radiating from its source
the
distance
from
that
source
the extent to which the radiation has been absorbed (or blocked, or shielded).
Particle model Because energy of an EM wave is quantized, in the particle model of EM radiation, a wave consists of discrete packets of energy, or quanta, called photons. The frequency of the wave is proportional to the magnitude of the particle's energy. Moreover, because photons are emitted and absorbed by charged particles, they act as transporters of energy. The energy per photon can be calculated by Planck's equation:
where E is the energy, h is Planck's constant, and f is frequency. This photon-energy expression is a particular case of the energy levels of the more general electromagnetic oscillator whose average energy, which is used to obtain Planck's radiation law, can be shown to differ sharply from that predicted by the equipartition principle at low temperature, thereby establishes a failure of equipartition due to quantum effects at low temperature[1]. As a photon is absorbed by an atom, it excites an electron, elevating it to a higher energy level. If the energy is great enough, so that the electron jumps to a high enough energy level, it may escape the positive pull of the nucleus and be liberated from the atom in a process called photoionisation. Conversely, an electron that descends to a lower energy level in an atom emits a photon of light equal to the energy difference. Since the energy levels of electrons in atoms are discrete, each element emits and absorbs its own characteristic frequencies. Together, these effects explain the absorption spectra of light. The dark bands in the spectrum are due to the atoms in the intervening medium absorbing different frequencies of the light. The composition of the medium through which the light travels determines the nature of the absorption spectrum. For instance, dark bands in the light emitted by a distant star are due to the atoms in the star's atmosphere. These bands correspond to the allowed energy levels in the atoms. A similar phenomenon occurs for emission. As the electrons descend to lower energy levels, a spectrum is emitted that represents the jumps between the energy levels of the electrons. This is manifested in the emission spectrum of nebulae. Today, scientists use this phenomenon to observe what elements a certain star is composed of. It is also used in the determination of the distance of a star, using the socalled red shift.
S-ar putea să vă placă și
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (400)
- EATSMSDocument9 paginiEATSMSDaniel EhieduÎncă nu există evaluări
- EsoDocument11 paginiEsoDaniel EhieduÎncă nu există evaluări
- SoberDocument4 paginiSoberDaniel EhieduÎncă nu există evaluări
- Soot - Docx Daniel EhieduDocument10 paginiSoot - Docx Daniel EhieduDaniel EhieduÎncă nu există evaluări
- The CalculusDocument515 paginiThe CalculusDaniel EhieduÎncă nu există evaluări
- Chapter 1bDocument42 paginiChapter 1bTigani A BrigdarÎncă nu există evaluări
- (Ebook - PDF) How To Crack CD Protections PDFDocument4 pagini(Ebook - PDF) How To Crack CD Protections PDFJorge ArévaloÎncă nu există evaluări
- Eogie Carter PDFDocument4 paginiEogie Carter PDFDaniel EhieduÎncă nu există evaluări
- Biography Steve JobsDocument64 paginiBiography Steve JobsAnonymous LnTsz7cpÎncă nu există evaluări
- American Relief PDFDocument2 paginiAmerican Relief PDFDaniel EhieduÎncă nu există evaluări
- Killing The Devil - ManuscriptDocument248 paginiKilling The Devil - ManuscriptDaniel EhieduÎncă nu există evaluări
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (588)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (73)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (266)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (121)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- Formula Sheet Ch16Document7 paginiFormula Sheet Ch16Amir HÎncă nu există evaluări
- A Technical Report On Geneva Stop Mechanism BY Afolabi Tolulope Caleb MATRIC NO: 18/30GD014Document14 paginiA Technical Report On Geneva Stop Mechanism BY Afolabi Tolulope Caleb MATRIC NO: 18/30GD014Josh AddyÎncă nu există evaluări
- Inverted PendulumDocument9 paginiInverted PendulumMahmoud Samir MahmoudÎncă nu există evaluări
- Kinematics FREE WORKSHEET/QUESTION BANK BY ANURAG TYAGI CLASSES (ATC)Document3 paginiKinematics FREE WORKSHEET/QUESTION BANK BY ANURAG TYAGI CLASSES (ATC)ANURAG TYAGI100% (2)
- Biomechanics: Lichun Lu, PHD Kenton R. Kaufman, PHD, Pe Michael J. Yaszemski, MD, PHDDocument16 paginiBiomechanics: Lichun Lu, PHD Kenton R. Kaufman, PHD, Pe Michael J. Yaszemski, MD, PHDIkre19Încă nu există evaluări
- Lecture Plans For MEC442-2017Document2 paginiLecture Plans For MEC442-2017hahahaÎncă nu există evaluări
- Elemen Mesin Rem PDFDocument46 paginiElemen Mesin Rem PDFM Fathin NaufalÎncă nu există evaluări
- Olar Cells Based On Quantum Dots: Multiple Exciton Generation and Intermediate BandsDocument10 paginiOlar Cells Based On Quantum Dots: Multiple Exciton Generation and Intermediate Bandstapasrout12Încă nu există evaluări
- Mom Ii Lab Experiment ListDocument1 paginăMom Ii Lab Experiment ListmansafÎncă nu există evaluări
- HW #7Document4 paginiHW #7c_sierra34Încă nu există evaluări
- TorsionalVibration PresentationDocument20 paginiTorsionalVibration PresentationValentin JonovÎncă nu există evaluări
- Revision Part2 PDFDocument23 paginiRevision Part2 PDFrawadÎncă nu există evaluări
- Shear Deformation of Thick BeamsDocument14 paginiShear Deformation of Thick BeamsboubressÎncă nu există evaluări
- Veres TheoryManualDocument35 paginiVeres TheoryManualgerardorochaÎncă nu există evaluări
- Performance Evaluation of Air PreheaterDocument8 paginiPerformance Evaluation of Air PreheaterAndria MatthewsÎncă nu există evaluări
- Retrocausality in Quantum Phenomena and Chemical EDocument10 paginiRetrocausality in Quantum Phenomena and Chemical Esophie MÎncă nu există evaluări
- Non-Destructive Assessment of ConcreteDocument34 paginiNon-Destructive Assessment of ConcreteKaan TekinturhanÎncă nu există evaluări
- FOUNDATION - Khmer PDFDocument143 paginiFOUNDATION - Khmer PDFMOMOÎncă nu există evaluări
- Multidisciplinary Optimization With Applications To Sonic-Boom MinimizationDocument24 paginiMultidisciplinary Optimization With Applications To Sonic-Boom MinimizationligÎncă nu există evaluări
- 5.1 Energy Changes in Chemical and Nuclear ReactionsDocument9 pagini5.1 Energy Changes in Chemical and Nuclear ReactionsMarissaÎncă nu există evaluări
- L&T Pile Intrigity & CHSL TestDocument101 paginiL&T Pile Intrigity & CHSL TestDevendra SinghÎncă nu există evaluări
- Beam On Elastic FoundationDocument8 paginiBeam On Elastic FoundationBoonme ChinnaboonÎncă nu există evaluări
- Chapter 3 PDFDocument46 paginiChapter 3 PDFRG RAJÎncă nu există evaluări
- Wave Optics QuestionDocument6 paginiWave Optics QuestionMayank KumarÎncă nu există evaluări
- The Superposition PrincipleDocument3 paginiThe Superposition PrincipleEza MarÎncă nu există evaluări
- Aqa MM1B W QP Jun07Document8 paginiAqa MM1B W QP Jun07jfarrell_ie5767Încă nu există evaluări
- Phy 150 Exp 1Document11 paginiPhy 150 Exp 1QhairunnissaÎncă nu există evaluări
- Cyclic Demand Spectrum: Earthquake Engineering & Structural Dynamics July 2002Document18 paginiCyclic Demand Spectrum: Earthquake Engineering & Structural Dynamics July 2002mikadituÎncă nu există evaluări
- DJJ30093 Chapter 1 Dem 3Document17 paginiDJJ30093 Chapter 1 Dem 3Sushantan SubramanyamÎncă nu există evaluări
- PhysicsDocument5 paginiPhysicsBrentÎncă nu există evaluări