Documente Academic
Documente Profesional
Documente Cultură
CHM 2210 Ps 16
Încărcat de
Jun LiDescriere originală:
Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
CHM 2210 Ps 16
Încărcat de
Jun LiDrepturi de autor:
Formate disponibile
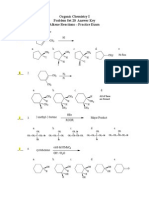
Organic Chemistry I Problem Set 16 Alcohols and Ethers Practice Exam
I. ____ Multiple Choice. Choose the best answer. 1. a. Which of these dehydrates most easily?
CH3
CH3 CH2 CH2 OH
CH3
b.
OH
c.
OH
d. ____ 2. a.
CH3 C CH2 OH CH3
e.
CH2OH
Which of these is oxidized most easily?
CH3-CHOH-CH 3
CH3
b.
OH
c.
CH3-CH2-O-CH 2-CH3
CH3
d. ____ 3. a. d. ____ 4.
CH3 C CH3 OH
e.
OH
Which of these is a reducing agent? CrO3 / H+ LiAlH4 b. KMnO4 e. O3 ? c. CH4 ?
CH3
c.
H+ / H2O
f. HCOOOH
CH3CH2MgBr + CH3OH b. CH3CH2OCH3
Na2Cr2O7 cool
CH3
a. CH3CH2CH3
CH3
d. CH3CH3
____
5.
CH3 C CH3 OH
a.
CH3 CH3 C CH2
CH3 CH3
b.
CH3 CH CH3
c.
CH3 C CH3 O
d.
CH3 C O C CH3 CH3 CH3
e. No reaction
____
6.
CH2
a. (BH3)2 b. H2O2 / OH-
a.
CH2OH
b.
CH3 OH
c.
d.
OH
____
7.
CH 3 CH 2 O CH 2 CH 3
a. LiAlH4 b. H 2O
a. CH3CH3 ____ 8. a.
b. CH3CH2OH
c. CH3CH2CH2CH3
d. No Reaction
An alcohol of formula C9H12O reacts with Na2Cr2O7 to form a compound with the formula C9H10O. The original alcohol might be.
OH
CH2 CH2 CH2 OH
CH3
b.
CH CH2-CH3
c.
C CH3
OH
d.
CH3 CH CH2OH
____
9. a.
Anisole
excess HI conc reflux
CH2I
b.
+ CH3I
c.
I + CH3OH
d.
OH
+ CH3I
e.
OH
+ CH3CH2I
____
10.
Br2 Fe
Mg ether
ethylene oxide
H+
CrO3/H+ cool
a. d.
benzoic acid phenylacetic acid
b.
acetophenone
c. phenylethyl ether f. styrene oxide
e. styrene
II.
Write the structures of the major products of the following reactions. If no reaction occurs, write N.R. 1.
H2SO 4 heat KMnO4 cold
OH
2.
a. Hg(CH 3COO -)2/H 2O b. NaBH
4
3.
CH3
a. Hg(CF3COO-) 2 / CH3OH b. NaBH4
4.
CH3 CH2 OH
Na
t-butCl
5.
CH3 CH2 CH2 OH
POCl3 heat
CH3COOOH
H+ / H2O
6.
PCC CH2 OH
7.
OH
CrO3 H+ / H2O cool
8.
acetic acid MgBr
9.
CH3 CH2 OH
NaOH
10.
CH3 CH2 OH
NaNH2
11.
CH3 C C CH3 CH2 OH
Na
12.
CH3 CH2 OH
Na
SH
13.
CH3 CH2 OH
NaH
14.
CH3 CH2 OH
CH3COOH H2SO 4, reflux
a. excess CH 3MgI b. H +
15.
MgBr
1. cyclohexanone 2. H3O+
16.
MgBr
1. CH3CH2CHO 2. H3O+
17.
MgBr
1. CO 2 2. H3O+
1. CH3OH 2. H3O+
18.
MgBr
19.
NaBH4 H2O / EtOH
O
20.
CH3 C CH3
O
1. LiAlH 4 2. H2O
1. NaOCH 3 / CH3OH
21.
H2C
CH CH3
2. H2O
22.
CH3 O CH3
1. LiAlH 4 2. H2O
23.
H2C
CH CH3
1. LiAlH 4 2. H2O CH3
24.
CH3 CH2 Br
+ CH3 C O CH3
III.
Answer the following questions: 1. When heated with H2SO4, neopentyl alcohol slowly dehydrates to form 2 alkenes. Show the steps in the mechanism, writing structures for all products of the steps. Be complete!
2.
An optically active compound of formula C9H12O2 produced the following when refluxed with KMnO4.
O HO C
O C OH
The original compound showed these properties as well:
C9H12O2
Na Br2 CrO3 / H+ cool
H2 liberated no rapid reaction C9H8O3
At this point these are at least two possible structures for the original compound. Show one of them.
3.
Using benzene, any straight chain alcohol, and ethylene oxide as sources of carbon, show how you would synthesize these compounds. (Hint Use Grignard rxns.) a.
CH3 CH2 O C CH3 CH3
b.
CH2 C O CH2 CH3
c.
O CH3 CH C CH2
S-ar putea să vă placă și
- Flowers From 1970Document93 paginiFlowers From 1970isla62% (21)
- Jodi Picoult - Where There's Smoke (Ebook)Document41 paginiJodi Picoult - Where There's Smoke (Ebook)Allen & Unwin100% (6)
- FIU ORGANIC CHEMISTRY PRACTICE EXAMDocument5 paginiFIU ORGANIC CHEMISTRY PRACTICE EXAMShaima MossamatÎncă nu există evaluări
- Carboxylic Acid Tutorial: Properties, Nomenclature & ReactionsDocument7 paginiCarboxylic Acid Tutorial: Properties, Nomenclature & Reactionsizabel50% (2)
- Chemistry Tutorial 1 Year Medicine and Dentistry Analytical ChemistryDocument6 paginiChemistry Tutorial 1 Year Medicine and Dentistry Analytical ChemistryTalal Ahmed Awad MohammedÎncă nu există evaluări
- Preparing Carbonyl CompoundsDocument6 paginiPreparing Carbonyl CompoundsVandana ReddyÎncă nu există evaluări
- Ffad 95919 BDocument5 paginiFfad 95919 BPreeti Gunthey DiwanÎncă nu există evaluări
- Chapter 17: Alcohols and PhenolsDocument29 paginiChapter 17: Alcohols and Phenols張湧浩Încă nu există evaluări
- Q-1 - Retest - Med II - 2020Document2 paginiQ-1 - Retest - Med II - 2020Kashmira WeerasekaraÎncă nu există evaluări
- Reaction and Preparation of Ethers and EpoxidesDocument2 paginiReaction and Preparation of Ethers and EpoxidesJAN JERICHO MENTOYÎncă nu există evaluări
- Esters: Formation, Naming, and UsesDocument22 paginiEsters: Formation, Naming, and UsesSania KhanÎncă nu există evaluări
- Chapter 21. Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution ReactionsDocument20 paginiChapter 21. Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions張湧浩Încă nu există evaluări
- Solution of Chemistry HSSC-II (3rd Set)Document11 paginiSolution of Chemistry HSSC-II (3rd Set)Ujala ShahidÎncă nu există evaluări
- Mechanism S.6 Chemistry-1Document17 paginiMechanism S.6 Chemistry-1alexlweerebugembeÎncă nu există evaluări
- Hydroxyacid Bai TapDocument10 paginiHydroxyacid Bai Tapvo anh nguyenÎncă nu există evaluări
- Chem Factsheet 17Document3 paginiChem Factsheet 17drdre12100% (1)
- Organic SynthesisDocument15 paginiOrganic SynthesisSamantha SwiftÎncă nu există evaluări
- Reactions and Preparations of Aldehydes and KetonesDocument5 paginiReactions and Preparations of Aldehydes and KetonesJAN JERICHO MENTOYÎncă nu există evaluări
- Test1 342 PracticeV1Document5 paginiTest1 342 PracticeV1Camha NguyenÎncă nu există evaluări
- CHM556 Assignment1Document4 paginiCHM556 Assignment1Saidin AhmadÎncă nu există evaluări
- BT Alcolhols Phenls and Ethers - ExerciseDocument20 paginiBT Alcolhols Phenls and Ethers - ExerciseSanjay KumarÎncă nu există evaluări
- MENTOY - Reaction and Preparation of Ethers and EpoxidesDocument2 paginiMENTOY - Reaction and Preparation of Ethers and EpoxidesJAN JERICHO MENTOYÎncă nu există evaluări
- Hydroxyl at I OnDocument60 paginiHydroxyl at I OngbgbkrishnaÎncă nu există evaluări
- La Síntesis Total de Colesterol: R. B. WoodwardDocument4 paginiLa Síntesis Total de Colesterol: R. B. WoodwardHector Martinez GregorioÎncă nu există evaluări
- Experiment 7Document2 paginiExperiment 7Wilma Parpana ParaderoÎncă nu există evaluări
- Aldehide: Metodele de ObţinereDocument7 paginiAldehide: Metodele de Obţinereisoscel34Încă nu există evaluări
- Road Maps Organic Chemistry Set 8 Eklavya @JEEAdvanced - 2024Document8 paginiRoad Maps Organic Chemistry Set 8 Eklavya @JEEAdvanced - 2024abhyudaipathwayÎncă nu există evaluări
- CHM 2210 Practice Exam 3Document8 paginiCHM 2210 Practice Exam 3Shaima MossamatÎncă nu există evaluări
- Carboxylic Acids: Structures, Nomenclature and ReactionsDocument41 paginiCarboxylic Acids: Structures, Nomenclature and ReactionsSazzad TanimÎncă nu există evaluări
- Carbonyl Compounds 1Document23 paginiCarbonyl Compounds 1Gowri ShankarÎncă nu există evaluări
- Chemistry Sample Test 11 PDFDocument4 paginiChemistry Sample Test 11 PDFdggdsgdsÎncă nu există evaluări
- Carboxylic Acids:: R-Cooh, R-Co HDocument29 paginiCarboxylic Acids:: R-Cooh, R-Co HRao Wazim AkramÎncă nu există evaluări
- Sample MCQ Organic Chemistry Sem II PSCH203 BacklogDocument4 paginiSample MCQ Organic Chemistry Sem II PSCH203 BacklogganesanneelamuruganÎncă nu există evaluări
- Chemistry Practice Test For University Exams 1Document5 paginiChemistry Practice Test For University Exams 1miyaskyeÎncă nu există evaluări
- Mechanism S.6 ChemistryDocument10 paginiMechanism S.6 Chemistryjordanmulumba34Încă nu există evaluări
- Exercise - IV Subjective Type: Solution Slot - 3 (Chemistry)Document2 paginiExercise - IV Subjective Type: Solution Slot - 3 (Chemistry)Priyanshu RajÎncă nu există evaluări
- Electrophilic Aromatic Substitution ReactionsDocument21 paginiElectrophilic Aromatic Substitution Reactions張湧浩Încă nu există evaluări
- Chemistry of Elements Tutorial/Assignment -2Document9 paginiChemistry of Elements Tutorial/Assignment -2Kumar KeshavÎncă nu există evaluări
- Carboxylic Acids:: R-Cooh, R-Co HDocument43 paginiCarboxylic Acids:: R-Cooh, R-Co HmacybnzÎncă nu există evaluări
- Ald Ketone Acid BCKDocument13 paginiAld Ketone Acid BCKVinayak BhatÎncă nu există evaluări
- CLS ENG 22 23 XII Che Target 5 Level 1 Chapter 13Document62 paginiCLS ENG 22 23 XII Che Target 5 Level 1 Chapter 13Harsh JakharÎncă nu există evaluări
- Aldehydes and Ketones-02 Solved ProblemsDocument13 paginiAldehydes and Ketones-02 Solved ProblemsRaju SinghÎncă nu există evaluări
- Chemistry HSSC-II Solution of 2nd Set Model Question PaperDocument15 paginiChemistry HSSC-II Solution of 2nd Set Model Question PaperIsha KhanÎncă nu există evaluări
- 2423 e 2Document24 pagini2423 e 2Agustin KurniatiÎncă nu există evaluări
- Alkylation of Enolate IonsDocument13 paginiAlkylation of Enolate IonsGabriel PekárekÎncă nu există evaluări
- Worksheet Chapter 4.IDocument2 paginiWorksheet Chapter 4.ImmmÎncă nu există evaluări
- Week 11Document4 paginiWeek 11sam cuadraÎncă nu există evaluări
- Aldehidos y Cetonas 2022 IiDocument5 paginiAldehidos y Cetonas 2022 IiJannina Carbajal VelásquezÎncă nu există evaluări
- Aldehydes, Ketones and Carboxylic Acids ChapterDocument1 paginăAldehydes, Ketones and Carboxylic Acids ChapterRishi KeshÎncă nu există evaluări
- Organic Chemistry I Reaction Sheet v2.1Document11 paginiOrganic Chemistry I Reaction Sheet v2.1Karl WilsonÎncă nu există evaluări
- ChemistryDocument34 paginiChemistryIvan Hoo Chean Yieng0% (1)
- 12 Chemistry Keypoints Revision Questions Chapter 12Document20 pagini12 Chemistry Keypoints Revision Questions Chapter 12sangam patraÎncă nu există evaluări
- Tutorial 6 AlcoholDocument5 paginiTutorial 6 Alcoholwan arifahÎncă nu există evaluări
- ALCOLDocument21 paginiALCOLthidaithanhÎncă nu există evaluări
- Saint Mary's University: Quiz On Balancing of Chemical EquationsDocument2 paginiSaint Mary's University: Quiz On Balancing of Chemical EquationsJuliannaViktoriiaÎncă nu există evaluări
- New Light Classes: 12B-Che Date-14/01/2022Document3 paginiNew Light Classes: 12B-Che Date-14/01/2022ShashwatÎncă nu există evaluări
- Practice Makes Perfect in Chemistry: Acids, Bases, and SaltsDe la EverandPractice Makes Perfect in Chemistry: Acids, Bases, and SaltsÎncă nu există evaluări
- Handbook of Coordination Catalysis in Organic ChemistryDe la EverandHandbook of Coordination Catalysis in Organic ChemistryÎncă nu există evaluări
- Practice Makes Perfect in Chemistry: Oxidation-ReductionDe la EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionEvaluare: 5 din 5 stele5/5 (1)
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersDe la EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersÎncă nu există evaluări
- UK Customs LeafletDocument13 paginiUK Customs LeafletOwen May-JonesÎncă nu există evaluări
- "I Want Some Methylamine!": Chapter FourteenDocument5 pagini"I Want Some Methylamine!": Chapter FourteenkomanieckizakapiorÎncă nu există evaluări
- Regular and Irregular Verbs PDFDocument5 paginiRegular and Irregular Verbs PDFDeyanid ALDANA CABRERA100% (1)
- Proteins FINALDocument3 paginiProteins FINALJin morarengÎncă nu există evaluări
- Stock Licores Liquidacion 01-09Document15 paginiStock Licores Liquidacion 01-09Victor Raul JuramaÎncă nu există evaluări
- WSET® Level 1 Award In: WinesDocument36 paginiWSET® Level 1 Award In: WinesNelson ChefsÎncă nu există evaluări
- Nice Chemical-Price List-2021-22Document84 paginiNice Chemical-Price List-2021-22Savitha NÎncă nu există evaluări
- Hydrocarbons Derivatives - Alcohols 13-18Document6 paginiHydrocarbons Derivatives - Alcohols 13-18Ahmed HammadÎncă nu există evaluări
- NewportCityCouncil March23Document313 paginiNewportCityCouncil March23NewportNow Dotcawm0% (1)
- Orgnic Conversions ForeignDocument10 paginiOrgnic Conversions ForeignSagarEricGaourÎncă nu există evaluări
- Tripel Karmeliet Clone: Fermentables Efficiency: Batch SizeDocument2 paginiTripel Karmeliet Clone: Fermentables Efficiency: Batch SizeKirk SantzÎncă nu există evaluări
- Stop Overdrinking BookDocument47 paginiStop Overdrinking Booklydia.gentry.dvmÎncă nu există evaluări
- Cocktails&Mocktails ListDocument19 paginiCocktails&Mocktails ListSameer KherÎncă nu există evaluări
- Recipes From Brooklyn Brew Shop's Beer Making BookDocument17 paginiRecipes From Brooklyn Brew Shop's Beer Making BookThe Recipe Club56% (9)
- Craft BeerDocument82 paginiCraft Beer9vadisÎncă nu există evaluări
- Cricket Club Seeks Permission for Youth EventDocument3 paginiCricket Club Seeks Permission for Youth EventDaniel DowdingÎncă nu există evaluări
- Wacker Oxidation - Chemistry LibreTextsDocument4 paginiWacker Oxidation - Chemistry LibreTextsasadÎncă nu există evaluări
- Als Practice Test Set eDocument35 paginiAls Practice Test Set eEdzel LloydÎncă nu există evaluări
- Vitamin B6 Derivative Synthesis and CharacterizationDocument3 paginiVitamin B6 Derivative Synthesis and CharacterizationAna Milena Aguilar CastellanosÎncă nu există evaluări
- Ingredients Method: Comments, Questions and TipsDocument4 paginiIngredients Method: Comments, Questions and TipsJohnny44aÎncă nu există evaluări
- Form 1 Retainer RecordDocument29 paginiForm 1 Retainer RecordGian Tristan Madrid100% (1)
- Krishna's Heartbreak & His Struggles With LoveDocument109 paginiKrishna's Heartbreak & His Struggles With LoveHarsh Aggarwal100% (1)
- Seminar On Alcohol Related Disorder and Its ManagementDocument41 paginiSeminar On Alcohol Related Disorder and Its ManagementSoumyasree.pÎncă nu există evaluări
- Stuctural Indefication - POC ExerciseDocument22 paginiStuctural Indefication - POC ExercisemikcÎncă nu există evaluări
- Beverage Technology: Innovative Solutions For Your SuccessDocument28 paginiBeverage Technology: Innovative Solutions For Your SuccessWinÎncă nu există evaluări
- 1901 Madeira and The Canary Islands, With The AzoresDocument463 pagini1901 Madeira and The Canary Islands, With The AzoresCarlos RojanoÎncă nu există evaluări
- Fermentation: What Is Anaerobic Respiration?Document22 paginiFermentation: What Is Anaerobic Respiration?NnekaÎncă nu există evaluări
- Reaction Paper: VIDEO REVIEW. Zucchero!Document3 paginiReaction Paper: VIDEO REVIEW. Zucchero!Julius CabinganÎncă nu există evaluări