Documente Academic
Documente Profesional
Documente Cultură
Cell Signalling
Încărcat de
Divya MohanDrepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Cell Signalling
Încărcat de
Divya MohanDrepturi de autor:
Formate disponibile
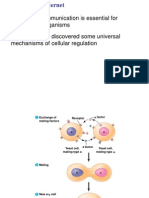
Cell-cell interactions
Cell Communication
Different Receptor Types
Ion channel-linked receptors
GPCR signaling
Enzyme linked receptors
Regulated proteolysis
INTRACELLULAR SIGNAL TRANSDUCTION:
A Journey from the Plasma Membrane to the Nucleus
INTRACELLULAR SIGNALING:
Signal Transduction
Cell membranes, as well as the cell cytoplasm and even
the cell nucleus, contain cell-specific receptors for which
are involved in outside-inside signaling,
i.e. signal transduction.
Ligands include hormones, growth factors, cytokines,
prostaglandins and proteases
Hormones are involved in a variety of metabolic
processes that maintain homeostasis
e.g. fuel metabolism. Particularly noteworthy in that
regard are glucagon, insulin and the catecholamines
(epinephrine and norepinephrine).
Growth factors are involved in mitogenesis, whereas
cytokines play critical roles in the differentiation,
proliferation and function of various cell lineages
Interaction of such ligands with their membrane, cell-
specific receptors or intracellular receptors causes
conformational changes in the receptor and, in many
instances receptor-associated cytoplasmic proteins.
Such events result in the initiation of a cascade of
important, but as yet incompletely understood, events
leading to e.g. enzyme activation, differentiation and/or
cell division
Extracellular signaling
molecules released by
cells occurs over distances
from a few microns -
autocrine (c) and paracrine
(b) signaling to several
meters in endocrine (a)
signaling. In some
instances, receptor proteins
attached to the membrane
of one cell interact directly
with receptors on an
adjacent cell (d).
FORCES DRIVING SELECTION OF CURRENT
MECHANISMS
Need for coordinated intercellular communication
Need to translate extracellular signals into series of
intracellular events, while allowing for specificity
Specificity Determinants:
1. Specific receptors on or in the target cells
recognize an appropriate ligand.
2. Specific response to receptor occupancy - effector
pathways
Diversity of intercellular communication is achieved
with hundreds of signaling molecules
Proteins, small peptides, amino acids, nucleotides,
steroids, fatty acid derivatives, and even dissolved
gases such as NO and CO
Intracellular receptors:
- signaling molecules include
steroid hormones, retinoids,
thyroxine, etc
- receptor-hormone complex
acts as a transcription factor
to alter transcription of certain
genes
Cell surface receptors:
- signaling molecules include
peptide hormones, catechol-
amines, insulin, growth
factors, cytokines, etc
binding, and subsequent events,
triggers an | or | in the
cytosolic concentration of
a second messenger; or the
activated, bound receptor acts
as a scaffold to recruit and
activate other intracellular
proteins
1. Each cell is programmed to
respond to specific
combinations of signaling
molecules.
2. Different cells can respond
differently to the same
chemical signal.
HORMONES - First class of signaling molecules
defined
Secreted from endocrine cells - specialized signaling cells that
control the behavior of an organism as a whole:
1.Differ from other intracellular mediators
2. Usually stimulate metabolic activities in tissues remote from the
secretory organ
3. Active at very low concentrations (pM - uM)
4. Response to hormonal signal comes as a direct and rapid result
of its secretion
5. Metabolized rapidly so effects are, in most instances, short-
lived, leading to rapid adaptations to metabolic changes
Categories:
1. Peptides or polypeptides - insulin, glucagon, growth
hormone, insulin-like growth factors, vasopressin,
prolactin.
2. Glycoproteins - follicle stimulating hormone (FSH),
thyroid stimulating hormone (TSH)
3. Steroids - glucocorticoids (aldosterone, cortisol),
steroids (progesterone,testosterone), retinoic acid
4. Amino acid derivatives - epinephrine,
norepinephrine, thyroxine, triidothyronine
AGONISTS vs. ANTAGONISTS
Agonist mimics a hormone in binding productively to
a receptor
Antagonist mimics a hormone stereochemically, but binds to the
receptor non-productively, inhibiting the action.
natural hormone
Agonist
Hormone
Antagonist
RECEPTOR CHARACTERISTICS
1. Participates in transduction of the signal from the
external messenger to some component of the
metabolic machinery
2. Has at least one additional functional site which is
altered by ligand binding (allosteric site)
3. Ligand binding to receptors is saturable, resembling
Michaelis-Menten kinetics
4. Ligand-receptor interaction characterized by tight
binding (Kd = pM - uM)
Intracellular Signaling Mediated by G protein-linked
Membrane Receptors
e.g. Glucagon, Epinephrine and Thrombin as signaling
molecules
1. Activates a chain of events alterations in
concentrations of signaling molecules; elaborate sets
of interacting molecules that can relay signals from
cell surface to the nucleus
2. Components: Receptor; Transducer (G protein):
Effector (membrane-bound enzyme);
Second messenger (e.g. cAMP); Response (cascade
of highly-regulated protein phosphorylations
H
3
N
+
C COO
CH
2
OH
H
serine (Ser)
H
3
N
+
C COO
CH OH
CH
3
H
threonine (Thr)
Many enzymes are regulated by covalent attachment
of phosphate, in ester linkage, to the side-chain
hydroxyl group of a particular amino acid residue
(serine, threonine, or tyrosine)
Protein OH + ATP Protein O P
O
O
+ ADP
P
i
H
2
O
Protein Kinase
Protein Phosphatase
A protein kinase transfers the terminal
phosphate of ATP to a hydroxyl group on a
protein.
A protein phosphatase catalyzes removal of
the Pi by hydrolysis
Phosphorylation may directly alter activity of an
enzyme, e.g., by promoting a conformational change.
Alternatively, altered activity may result from binding
another protein that specifically recognizes a
phosphorylated domain.
E.g., 14-3-3 proteins bind to domains that include
phosphorylated Ser or Thr in the sequence
RXXX[pS/pT]XP, where X can be different amino
acids.
Binding to 14-3-3 is a mechanism by which some
proteins (e.g., transcription factors) may be retained
in the cytosol, & prevented from entering the
nucleus
Protein kinases and phosphatases are
themselves regulated by complex signal
cascades. For example:
Some protein kinases are activated by Ca++-
calmodulin.
Protein Kinase A is activated by cyclic-AMP
(cAMP).
N
N
N
N
NH
2
O
OH
O
H
H
H
H
2
C
H
O
P
O
O-
1'
3'
5'
4'
2'
cAMP
Adenylate Cyclase
(Adenylyl Cyclase)
catalyzes:
ATP cAMP + PPi
Binding of certain hormones
(e.g., epinephrine) to the
outer surface of a cell
activates Adenylate Cyclase
to form cAMP within the
cell.
Cyclic AMP is thus
considered to be a second
messenger.
N
N
N
N
NH
2
O
OH
O
H
H
H
H
2
C
H
O
P
O
O-
1'
3'
5'
4'
2'
cAMP
Phosphodiesterase
enzymes catalyze:
cAMP + H2O AMP
The phosphodiesterase
that cleaves cAMP is
activated by
phosphorylation
catalyzed by Protein
Kinase A.
Thus cAMP stimulates its
own degradation, leading
to rapid turnoff of a
cAMP signal.
RECEPTOR: typically a seven transmembrane domain, integral
membrane protein
|-adrenergic receptor
All G protein-coupled receptors resemble the |-adrenergic receptor
in their amino acid sequence and membrane topography.
Signal Transduction Pathway Activation of Adenylate Cyclase by Gs
Receptor: 7 TM domain integral membrane protein
Transducer: Gs
Effector: adenylate
cyclase
Second messenger:
cAMP
Response: PKA
regulated
protein
phosphorylations
Cycle of G protein dissociation/association:
Activation of G proteins involves GTP
displacement of GDP bound to the o subunit
and dissociation of the complex from the |
subunits, an exchange facilitated by a GEF
protein (guanine nucleotide exchange factor)
specific for Gs.
The active o subunit binds to and activates/
stimulates membrane associated adenylate
cyclase which catalyzes the conversion of ATP to
cyclic AMP (cAMP).
The GTPase activity associated with the o subunit
slowly hydrolyzes the bound GTP to GDP which is
facilitated by a GAP (GTPase activating protein)
specific for Gs.
The o subunit with GDP bound reassociates
with the | subunits, and membrane
associated adenylate cyclase returns to its
basal activity level
G-protein-Coupled Receptors may dimerize or form
oligomeric complexes within the membrane.
Ligand binding may promote oligomerization, which may
in turn affect activity of the receptor.
Various GPCR-interacting proteins (GIPs) modulate
receptor function. Effects of GIPs may include:altered
ligand affinity receptor dimerization or oligomerization
control of receptor localization, including transfer to or
removal from the plasma membrane promoting close
association with other signal proteins
Characteristics of G proteins
1. G protein is an o| trimeric protein which binds guanine
nucleotides.
2. They function to couple integral membrane receptors to target
membrane-bound enzymes.
3. They can be considered molecular switches wherein
o|
GDP
(inactive) o
GTP
(active) + |
4. The dissociated o subunit expresses GTPase activity
5. GTPS blocks GTPase activity of oGTP
o
i
| with
GDP bound
Region that changes
conformation when
GTP is hydrolyzed
Activation of cAMP-dependent protein kinase (PKA)
cAMP binds to the PKA regulatory subunits conformational changes, which
causes their dissociation from the catalytic subunits kinase activation.
Release of the catalytic subunits requires the binding of more than two cyclic
AMP molecules greatly sharpening the response of the kinase to changes in
[cAMP].
PKA is a Ser/Thr kinase with discrete substrate specificity, thus facilitating a
cascade of highly regulated protein phosphorylations.
The Whole Signaling
Network related to cAMP
Signal Transduction Pathway Activation of
Adenylate Cyclase by Gs
Down stream effects:
1. Phosphorylation of regulatory enzymes of
metabolic pathways.
2. Increase transcriptionof certain proteins via
phosphorylation of CREB leading to | protein syn-
thesis of target proteins.
Activated PKA enters the
nucleus and phosphorylates
CREB (cAMP Response
Element Binding protein).
Once phosphorylated, CREB
recruits the coactivator CBP
(CREB Binding Protein).
This complex binds to the
CREB-binding element to
stimulate gene transcription
How Increased Intracellular cAMP Leads to Increased Gene Transcription
Signal Transduction Pathway Activation of Adenylate Cyclase by Gs
Early off signals:
1. Gso-associated GTPase
activity GTP hydrolysis
to GDP leading to re-
association of GsGDP
and dissociation of the
hormone/receptor complex
Down stream off signals:
1. cAMP hydrolyzed
by phosphodiesterase
2. Ser/Thr phosphatases
dephosphorylate the
phosphorylated target
proteins.
Desensitization or Endocytosis of GPCRs Effected by Phosphorylation
1. The ligand activated receptor can be phosphorylated on select Ser/Thr
2. residues by GRK (e.g. BARK - | adrenergic receptor kinase). These
phosphorylated residues
provide a docking site for arrestin resulting in inactivation/desensitization.
2. In some instances, arrestin binding targets the receptor for clathrin-dependent
endocytosis.
3. In addition, if the occupied GPCR leads to | cAMP, the receptor can also be
phosphorylated by PKA leading to its inactivation/densensitization.
Clathrin-dependent Receptor-mediated endocytosis
Gs vs. Gi
Regulation of Adenylate Cyclase Activity
Gs stimulates adenylate cyclase
Gi inhibits adenylate cyclase
e.g. epinephrine can increase or decrease
intracellular cAMP concentrations,depending
upon the receptor to which it binds
| adrenergic receptors couple to Gs, whereas
o2 adrenergic receptors couple to Gi
Vasopressin binding to its GPCR activates Gs | cAMP and activation
of PKA.
PKA phosphorylates various proteins the ultimate aggregation of
microtubular subunits, which insert as water channels in the luminal
plasma membrane to increase the reabsorption of water by free diffusion.
Inhibition of Gs and Gi by Bacterial Toxins
Cholera toxin effects on Gs:
ADP ribosylation of an Arg
residue in the os subunit of
Gs inhibition of associated
GTPase activity
Pertussis toxin effects on Gi:
ADP ribosylation of a Cys
residue in the oi subunit of Gi
an inability to inhibit
adenylate cyclase activity.
Thus, both toxins cause
increased intracellular cAMP
concentrations!
Gs vs Gi vs Gq
Gs and Gi coupled to adenylate cyclase A
[cAMP]
Gq coupled to phospholipase C A [Ca2+]
INTRACELLULAR Ca2+ AS A SECOND MESSENGER
Cells must work very hard to maintain low intracellular [Ca2+]
in order for| Ca2+ to result in effective intracellular signaling.
Cellular mechanisms that maintain very low intracellular Ca2+
concentrations
A: Ca2+ is actively pumped out of the cytosol.
B: Ca2+ is pumped into the ER and mitochondria.
Three Types of Inositol phospholipids
PI, PI(4)P, PI(4,5)P2
Phospholipase C- |
(PLC-|) Produces
DAG
(diacylglycerol) and
IP3 (inositol 1,4,5-
trisphosphate (IP3))
Gq->PLC-|
Gq signaling pathways and Calcium
Phospholipase C| (PLC|)-catalyzed hydrolysis of PIP2 Formation of 2
second messengers
IP3 release of Ca2+ from intracellular stores by binding to an IP3-gated
Ca2+ channel in the endoplasmic reticulum.
DAG with released Ca2+ and membrane-associated phosphatidylserine
activates Protein Kinase C (PKC - a Ser/Thr kinase). PKC directly
phosphorylates intracellular proteins some of which | gene transcription
Phosphoinositide-activated Second Messenger System
| Intracellular [Ca2+]
Ca2+ Second Messenger System: Release from
intracellular stores subsequent to PLC|-catalyzed hydrolysis
of PIP2
1)Must be distinguished from cAMP-induced effects where
cAMP activates a variety of Ca2+ channels | Ca2+ influx
from extracellular milieu (e.g. |-adrenergic receptor
occupancy in muscle cells | Ca2+ influx | rate and
force of heart beat
2)Increased intracellular [Ca2+] - Third messenger: Immediate
vs. Sustained responses Ca2+ binds to its ubiquitous
intracellular receptor, calmodulin, thereby a) activating
Ca2+/calmodulin-dependent kinases (CaM kinases), Ser/Thr
kinases b) that have their own sets of substrate proteins,
some of which when phosphorylated can | or | gene
transcription.
Increased Intracellular Ca2+: A Third Messenger
Calmodulin in a ubiquitous intracellular Ca2+ receptor
Ca2+/calmodulin (CaM) complexes activate CaM-dependent
kinases (e.g. CaM-kinase II)
Fertilization of an egg by a sperm triggering an increase in
cytosolic Calcium
3 major types of calcium channels:
Voltage dependent Ca channels on plasma membrane
IP3-gated Ca release channels on ER membrane
Ryanodine receptor on ER membrane
Calcium uptake and deprivation
Na/Ca exchanger on plasma membrane, 2. Ca pump on ER
membrane, 3. Ca binding molecules, 4. Ca pump on Mitochondia
Enzyme-Linked Cell Surface Receptors
*Receptor Tyrosine Kinase
*Tyrosine kinase associated receptors
*Receptor-like tyrosine phosphatase
*Receptor serine/threonine kinase
Receptor guanylyl cyclase
Histidine like associated receptor
Receptor Tyrosine Kinases
(RTKs)
Seven subfamilies of receptor tyrosine kinases
Three ways in which signaling proteins can
cross-link receptor chains
1. dimer, 2. monomer but brought together
by proteoglycan, 3. cluster on membrane
The importance of
receptor
oligomerization
The docking of signaling molecules at RTK
The binding of SH2-containing intracellular signaling
proteins to an activated PDGF receptor
RTK Signaling: Ras
Pathway
The regulation of Ras activity, a famous downstream
molecule of RTK responsible for cancer development
The activation of Ras by RTK signaling
The MAP-kinase regulated by Ras
RTK Signaling: PI3K Pathway
The inositol phospholipids generated by PI3K
Intracellular Signaling Pathways activated by RTKs
and GPCRs
1. Protein kinase Summary
Enzyme-linked Receptor Signaling Summary
1. receptor types
2. RTK and its signaling: Ras and PI3K
3. Tyrosine kinase associated receptors and
Receptor-like tyrosine phosphatase
4. Receptor serine/threonine kinase, TGF-b
and Smad
Notch and Delta interaction
Lateral inhibition
The inhibitory pathway of Notch
Proteolysis-mediated
Summary
1. GPCR signaling: PKA and Calcium
2. Enzyme-linked Receptor signaling: RTK->Ras
and PI3K
3. Proteolysis-mediated signaling pathways
S-ar putea să vă placă și
- Bacterial TransposonsDocument36 paginiBacterial TransposonsShweta SinghÎncă nu există evaluări
- Dna Genes Chromosomes 2011Document65 paginiDna Genes Chromosomes 2011Suliman GarallehÎncă nu există evaluări
- Cell SignallingDocument37 paginiCell SignallingSreekarWunnava100% (1)
- Cell Cycle and Cell DivisionDocument12 paginiCell Cycle and Cell DivisionMajid Al-hachami100% (1)
- Study Guide - Gene Regulation (CH 16)Document5 paginiStudy Guide - Gene Regulation (CH 16)jaysusgagaÎncă nu există evaluări
- TransposonesDocument6 paginiTransposonesPiero PaoloÎncă nu există evaluări
- Cell Signaling in Space and Time:: Where Proteins Come Together and When They're ApartDocument20 paginiCell Signaling in Space and Time:: Where Proteins Come Together and When They're ApartHumbertoÎncă nu există evaluări
- Cell Cycle RegulationDocument4 paginiCell Cycle RegulationSomÎncă nu există evaluări
- RetrovirusDocument23 paginiRetrovirusali haidarÎncă nu există evaluări
- Regulation of the Cell Cycle by Protein KinasesDocument5 paginiRegulation of the Cell Cycle by Protein KinasesmohsinÎncă nu există evaluări
- Signal Transduction Mechanisms and Cellular ResponsesDocument39 paginiSignal Transduction Mechanisms and Cellular ResponsesBellony Sanders100% (1)
- Intracellular SignallingDocument45 paginiIntracellular Signallingsana iqbalÎncă nu există evaluări
- DR Shazia RashidDocument21 paginiDR Shazia RashidShubham agrayÎncă nu există evaluări
- Gene Regulation: Mrs. Ofelia Solano SaludarDocument39 paginiGene Regulation: Mrs. Ofelia Solano SaludarmskikiÎncă nu există evaluări
- Dna RepairDocument20 paginiDna RepairEaron Van JaboliÎncă nu există evaluări
- Chromatin RemodellingDocument234 paginiChromatin Remodellingplastioid4079Încă nu există evaluări
- In-Depth Steps Towards Nucleic Acid and Protein SynthesisDocument21 paginiIn-Depth Steps Towards Nucleic Acid and Protein SynthesisGbenga AjaniÎncă nu există evaluări
- Regulation of Gene Expression in Prokaryotes: © John Wiley & Sons, IncDocument65 paginiRegulation of Gene Expression in Prokaryotes: © John Wiley & Sons, IncRoberto CastroÎncă nu există evaluări
- General Translation MechanismDocument15 paginiGeneral Translation MechanismAishwarya KashyapÎncă nu există evaluări
- Chapter 26 Phylogeny and The Tree of LifeDocument41 paginiChapter 26 Phylogeny and The Tree of LifeSheerin Sulthana100% (1)
- Cell Junction Types and Functions in 40 CharactersDocument30 paginiCell Junction Types and Functions in 40 CharactersNavodit GoelÎncă nu există evaluări
- Unit 1: Structural GenomicsDocument4 paginiUnit 1: Structural GenomicsLavanya ReddyÎncă nu există evaluări
- Module-3 (Theory) Upstream and Down Stream Component of A Fermentation Process PDFDocument6 paginiModule-3 (Theory) Upstream and Down Stream Component of A Fermentation Process PDFAnonymous AgGBWdÎncă nu există evaluări
- Signal Transduction in Cells: An Overview of Key ConceptsDocument25 paginiSignal Transduction in Cells: An Overview of Key ConceptsSadaf BegÎncă nu există evaluări
- A Brief Review of Plants Having Anti Cancer PropertyDocument8 paginiA Brief Review of Plants Having Anti Cancer PropertysharammaÎncă nu există evaluări
- FASTADocument33 paginiFASTAAnton MelcherÎncă nu există evaluări
- Gene Regulation in Prokaryotes-1Document17 paginiGene Regulation in Prokaryotes-1Fasiha Mushadi100% (1)
- Crispr Cas HajarDocument21 paginiCrispr Cas HajarHajira Fatima100% (1)
- Biological DatabaseDocument19 paginiBiological DatabaseMahesh Yadav100% (8)
- Cellular and Molecular Mechanisms of Inflammation: Receptors of Inflammatory Cells: Structure—Function RelationshipsDe la EverandCellular and Molecular Mechanisms of Inflammation: Receptors of Inflammatory Cells: Structure—Function RelationshipsCharles G. CochraneÎncă nu există evaluări
- 5 Proteomics and Metabolomics 23Document59 pagini5 Proteomics and Metabolomics 23CARMEN MERELLO AZPEITIAÎncă nu există evaluări
- Mechanisms of Hormone Action NotesDocument3 paginiMechanisms of Hormone Action Notesapi-390361165Încă nu există evaluări
- Chemical Modulators of Protein Misfolding and Neurodegenerative DiseaseDe la EverandChemical Modulators of Protein Misfolding and Neurodegenerative DiseaseÎncă nu există evaluări
- CH 11 PPT Cell Communication 1Document77 paginiCH 11 PPT Cell Communication 1api-270681964Încă nu există evaluări
- Cell Communication - DADocument66 paginiCell Communication - DAMuhammad Ardiansyah MÎncă nu există evaluări
- Cell Adhesion MoleculesDocument14 paginiCell Adhesion MoleculesSecret Agent100% (1)
- Chromatin RemodelingDocument5 paginiChromatin RemodelingRohit GargÎncă nu există evaluări
- cDNA Libraries and Gene CloningDocument8 paginicDNA Libraries and Gene CloningRoberto RomeroÎncă nu există evaluări
- Mechanism of Protein Targeting Into Endoplasmic ReticulumDocument24 paginiMechanism of Protein Targeting Into Endoplasmic ReticulumRebati Raman PandaÎncă nu există evaluări
- Comet AssayDocument15 paginiComet AssayarunsaintÎncă nu există evaluări
- L 10 Post Transcriptional ModificationDocument33 paginiL 10 Post Transcriptional ModificationsÎncă nu există evaluări
- Second Messenger Signaling SystemsDocument42 paginiSecond Messenger Signaling SystemsMirza Shaharyar BaigÎncă nu există evaluări
- DNA Topology Supercoiling and Linking PDFDocument5 paginiDNA Topology Supercoiling and Linking PDFmanoj_rkl_07Încă nu există evaluări
- Cell Communication PDFDocument109 paginiCell Communication PDFediaz_956003100% (1)
- Regulation of Histidine and Hut OperonsDocument11 paginiRegulation of Histidine and Hut Operonsaditi_joshee419Încă nu există evaluări
- Free Radical Theory of Aging: Reactive Oxygen Species, Mitochondria and Aging ProcessDocument57 paginiFree Radical Theory of Aging: Reactive Oxygen Species, Mitochondria and Aging ProcessdvdmegaÎncă nu există evaluări
- Ligand Cell Signaling PathwaysDocument117 paginiLigand Cell Signaling PathwaysShoaib MomenÎncă nu există evaluări
- Signal TransductionDocument38 paginiSignal Transductionjuju100% (2)
- Fundamental GeneticsDocument14 paginiFundamental GeneticsjuvemaÎncă nu există evaluări
- Eukaryotic TranscriptionDocument16 paginiEukaryotic TranscriptionKunal DuttaÎncă nu există evaluări
- A Plasmid Is A Small DNA Molecule Within A Cell That Is Physically Separated From A Chromosomal DNA and Can Replicate IndependentlyDocument5 paginiA Plasmid Is A Small DNA Molecule Within A Cell That Is Physically Separated From A Chromosomal DNA and Can Replicate Independentlyyaqoob008Încă nu există evaluări
- PlasmidsDocument53 paginiPlasmidsPrerana SikarwarÎncă nu există evaluări
- Genome Organization in ProkaryotesDocument8 paginiGenome Organization in ProkaryotesVijay Kishore75% (4)
- Specific Host Defenses: The Immune ResponseDocument54 paginiSpecific Host Defenses: The Immune Responseadyaly44Încă nu există evaluări
- Characteristics and Genotyping (Semi-Automated and Automated), Apparatus Used in GenotypingDocument45 paginiCharacteristics and Genotyping (Semi-Automated and Automated), Apparatus Used in GenotypingKhalid HameedÎncă nu există evaluări
- Transcription FactorsDocument25 paginiTranscription FactorsPriya.RÎncă nu există evaluări
- Yeast Transgenic PlantsDocument5 paginiYeast Transgenic PlantsTooba Iqbal67% (6)
- Functional and Structural Properties of Natural BiomembranesDocument7 paginiFunctional and Structural Properties of Natural BiomembranesAhsan RazaÎncă nu există evaluări
- Immunohistochemistry Principles Uses and MethodsDocument10 paginiImmunohistochemistry Principles Uses and MethodsKailash PrajapatÎncă nu există evaluări
- Biochemistry of NeurotransmittersDocument7 paginiBiochemistry of NeurotransmittersHyphophysis 2015Încă nu există evaluări
- Amino Acid Sequencing Techniques and Protein Structure DeterminationDocument32 paginiAmino Acid Sequencing Techniques and Protein Structure Determinationsuu55Încă nu există evaluări
- GE IV TH SemDocument185 paginiGE IV TH Semrahul vivekÎncă nu există evaluări
- G-Protein Coupled ReceptorsDocument8 paginiG-Protein Coupled ReceptorsHyunji KimÎncă nu există evaluări
- Fluid Mechanics, Bernoulli's Principle and Equation of ContinuityDocument10 paginiFluid Mechanics, Bernoulli's Principle and Equation of ContinuitySheerin SulthanaÎncă nu există evaluări
- J. Gen. Appl. Microbiol., 15, 387-398 (1969)Document12 paginiJ. Gen. Appl. Microbiol., 15, 387-398 (1969)Sheerin SulthanaÎncă nu există evaluări
- Trade-Related Intellectual Property Rights (Trips)Document20 paginiTrade-Related Intellectual Property Rights (Trips)Sheerin SulthanaÎncă nu există evaluări
- Unesco - Eolss Sample Chapters: Lysine Biosynthesis in Bacteria - An Unchartered Pathway For Novel Antibiotic DesignDocument11 paginiUnesco - Eolss Sample Chapters: Lysine Biosynthesis in Bacteria - An Unchartered Pathway For Novel Antibiotic DesignSheerin SulthanaÎncă nu există evaluări
- Bioethics Timeline and ControversiesDocument18 paginiBioethics Timeline and ControversiesSheerin SulthanaÎncă nu există evaluări
- BCH440 (8) 32-33Document20 paginiBCH440 (8) 32-33Sheerin SulthanaÎncă nu există evaluări
- Appl. Microbiol. 1968 Kato 1200 6Document8 paginiAppl. Microbiol. 1968 Kato 1200 6Sheerin SulthanaÎncă nu există evaluări
- Human Genome ProjectDocument57 paginiHuman Genome ProjectDhaval ParekhÎncă nu există evaluări
- A Business-Oriented Overview of Intellectual Property For Law StudentsDocument16 paginiA Business-Oriented Overview of Intellectual Property For Law StudentsSheerin SulthanaÎncă nu există evaluări
- Trade-Related Intellectual Property Rights (Trips)Document20 paginiTrade-Related Intellectual Property Rights (Trips)Sheerin SulthanaÎncă nu există evaluări
- Analysis On The Impact of Madrid Protocol For The Economies of Developing CountriesDocument62 paginiAnalysis On The Impact of Madrid Protocol For The Economies of Developing CountriesSheerin Sulthana0% (1)
- 32721Document33 pagini32721prasadbheemÎncă nu există evaluări
- Biological Safety Levels: Endia Ford Lori Gladney Izabella OsakweDocument34 paginiBiological Safety Levels: Endia Ford Lori Gladney Izabella OsakweSheerin Sulthana100% (1)
- Elements of Biosafety: Janet Peterson Jpeterso@accmail - Umd.edu 405-3975Document28 paginiElements of Biosafety: Janet Peterson Jpeterso@accmail - Umd.edu 405-3975Sheerin SulthanaÎncă nu există evaluări
- Radioimmunoassay: Advanced Research Techniques in Basic Medical SciencesDocument23 paginiRadioimmunoassay: Advanced Research Techniques in Basic Medical SciencesKiran FriendooÎncă nu există evaluări
- Internet and NetworkingDocument42 paginiInternet and Networkingآصف رضاÎncă nu există evaluări
- ATCCDocument3 paginiATCCSheerin SulthanaÎncă nu există evaluări
- Dimensions of Product Quality ManagementDocument5 paginiDimensions of Product Quality ManagementSheerin SulthanaÎncă nu există evaluări
- Emerging: Brewing Is The Production ofDocument16 paginiEmerging: Brewing Is The Production ofSheerin SulthanaÎncă nu există evaluări
- Lecture 21 - Respiratory SystemDocument59 paginiLecture 21 - Respiratory SystemSheerin SulthanaÎncă nu există evaluări
- Foodborne IllnessDocument13 paginiFoodborne IllnessSheerin SulthanaÎncă nu există evaluări
- The Range of Quality: Quality of Design Quality of Materials Quality of ProductsDocument4 paginiThe Range of Quality: Quality of Design Quality of Materials Quality of ProductsKin SkyeÎncă nu există evaluări
- 06iso Allo Idio09Document18 pagini06iso Allo Idio09Sheerin SulthanaÎncă nu există evaluări
- Foods Poli AgeDocument23 paginiFoods Poli AgeSheerin SulthanaÎncă nu există evaluări
- Pricing ProblemsDocument2 paginiPricing ProblemsSheerin SulthanaÎncă nu există evaluări
- 10 1371-Journal Pone 0065654 g003Document1 pagină10 1371-Journal Pone 0065654 g003Sheerin SulthanaÎncă nu există evaluări
- (WWW - Entrance Exam - Net) Animal BiotechDocument34 pagini(WWW - Entrance Exam - Net) Animal BiotechSheerin SulthanaÎncă nu există evaluări