Documente Academic
Documente Profesional
Documente Cultură
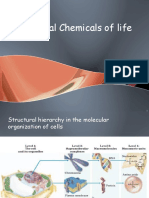
Biochemistry of Water, Carbon Compounds, and Macromolecules
Încărcat de
Ana AyalaDescriere originală:
Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Biochemistry of Water, Carbon Compounds, and Macromolecules
Încărcat de
Ana AyalaDrepturi de autor:
Formate disponibile
Biochemistry: Water and Organic Compounds
Modern Biology Chapter 3
Water
Formed by covalent bonds (atoms share electrons)
Atoms dont share equallywater is POLAR
Oxygen end is slightly negative (O is electron hog) Hydrogen ends are slightly positive POLARITY is why water is a good solvent
Dissolves lots of different compounds
Hydrogen Bonding in Water
Polarity causes water to be attracted to each other H-bond: attraction that holds water molecules together Responsible for cohesion (sticking together) that produces surface tension
Cohesion and adhesion (attraction between unlike substances) responsible for capillarity (water moves up through stems)
Water has to gain/lose lots of energy to change temp.
This energy initially goes into breaking H-bonds This is why a pot of water on the stove isnt hot right away
Carbon Compounds
Organic compounds
Contain carbon atoms covalently bonded to each other and to other elements (H, O, N, usually) Needs 8 to be stableshares its electrons with other atoms
Carbon has 4 electrons in its outer E-level
Able to form straight chains, branched chains, or rings
Examples of Carbon bonds
Propane
Ethylene (plant hormoneripens fruit)
2-methylpropane (used in refrigeration and petrochemical industry)
Glucose
Functional Groups
Cluster of atoms that influence the properties of the molecule they are attached to.
Ex: -OH (hydroxl group)
Alcohols contain hydroxyl groups Makes alcohols polar
Allows them to dissolve in water, have H-bonds like water
Carbon Molecules
Monomers
Condensation Rxn
Small and simple; single building block Made of repeated, linked monomers
Polymers
Links monomers together AKA dehydration synthesis Forms water
Macromolecule
Hydrolysis Rxn
Made of linked polymers
Uses water to break polymers apart
Condensation/Hydrolysis
ATP
Adenosine Triphosphate
Energy molecule used by the body Broken down into ADP (releases P) ADP + P ATP Continuous Cycle
Carbohydrates
Made of C, H, O Ratio of H to O is 2:1 Sugars! Used for quick energy
Monosaccharides
Building blocks of all carbs (CH2O)n is generic formula Simple sugars Most common are glucose, fructose, galactose
Isomers: same formula, different structure
Carbohydrates
Disaccharides
Formed from 2 monosaccharides
Condensation reaction Ex: sucrose
Polysaccharides
Formed from 3+ monosaccharides
Condensation reaction
Glycogen: storage in animals (used for energy) \ Starch: storage in plants Cellulose: in plant cell walls (gives them rigidity, crunch)
Lipids
Fats! Large, non-polar; do not dissolve in water Have more C, H than O Responsible for storing energy
Lipids
Fatty Acids
Building blocks of lipids Straight carbon chain with carboxyl group (COOH) on one end (makes that end polar) Carboxyl end head = hydrophilic
Water-Loving Water-Fearing Saturated: all C-C bonds are single bonds (is full of Hs) Unsaturated: contains some double bonds
Carbon chain tail = hydrophobic
Can be Saturated or Unsaturated
Lipids
Lipids
Triglycerides
Waxes
3 Fatty Acids attached to 1 Glycerol molecule
Saturated: solid at room temp (shortening) Unsaturated: liquid at room temp (oils, found in plant seeds/fruits)
Waterproof Form protective coating on plant leaves
Steroids
Phospholipids
4 fused carbon rings w/ functional groups Hormones
2 FAs attached to 1 Glycerol
Major component of cell membrane (lipid bilayer)
Cholesterol Testosterone Etc.
Proteins
Made of C, H, O, and N Building blocks are Amino Acids
Amino Acids (20) are almost identical
Difference is the R-group (functional group)
Di-peptide: chain of 2 Amino Acids Polypeptide: lots of AAs linked together Bond that holds AAs together is peptide bond
Proteins
Amino Acid Structure
Enzymes
Proteins Act as catalyst for many reactions that occur in the body Substrate: what the enzyme acts on Product(s): what is formed from the rxn
Enzymes
Are not used up during the reaction Are not changed during the reaction
Can do the job again and again Specific shape, must fit perfectly for rxn to occur
Lock and Key fit
Enzymes can be denatured (destroyed) when:
Change in temperature (too high) Change in pH
Nucleic Acids
Used by cells to store hereditary information
DNA
Deoxyribonucleic acid Ribonucleic acid
RNA
Made of building blocks called nucleotides
5-carbon sugar Phosphate group Nitrogen base
S-ar putea să vă placă și
- Molecules of LifeDocument38 paginiMolecules of LifeAmanda ArceoÎncă nu există evaluări
- BIO 22 MODULE 1 - Chemical Basis of LifeDocument14 paginiBIO 22 MODULE 1 - Chemical Basis of LifeBryan DGÎncă nu există evaluări
- Chapter 02 Winter 2020Document53 paginiChapter 02 Winter 2020LESLI RODRIGUEZ BENDEZUÎncă nu există evaluări
- Walang MakakaseethisDocument3 paginiWalang MakakaseethisAlissa YangÎncă nu există evaluări
- Biology Notes Unit 1Document40 paginiBiology Notes Unit 1MirzaAteeqAhmedBaigÎncă nu există evaluări
- AP Bio Unit 1 Study GuideDocument2 paginiAP Bio Unit 1 Study GuideEllie GriffinÎncă nu există evaluări
- AQA Module All Revision NotesDocument82 paginiAQA Module All Revision NotesRS JÎncă nu există evaluări
- Carbohydrates SlidesDocument75 paginiCarbohydrates SlidesShiva PratheekÎncă nu există evaluări
- As 2 Biological MoleculesDocument29 paginiAs 2 Biological MoleculesisaacÎncă nu există evaluări
- Biological Molecules: Reducing Sugars TestsDocument5 paginiBiological Molecules: Reducing Sugars TestsShinuk KangÎncă nu există evaluări
- Smackslidecom Powerpoint Temp 6032625eb534aDocument54 paginiSmackslidecom Powerpoint Temp 6032625eb534aQuang MinhÎncă nu există evaluări
- 3 Essential Chemicals of Life PPTDocument82 pagini3 Essential Chemicals of Life PPTBhoni KumariÎncă nu există evaluări
- Biochemistry of CellsDocument79 paginiBiochemistry of CellsketakeeÎncă nu există evaluări
- AQA As-Level Biology Revision Notes - New SpecDocument38 paginiAQA As-Level Biology Revision Notes - New SpecjimboÎncă nu există evaluări
- Biology Revision NotesDocument42 paginiBiology Revision Noteshumza bhattiÎncă nu există evaluări
- Clase 2 Chemistry of CellDocument73 paginiClase 2 Chemistry of Cellediaz_956003Încă nu există evaluări
- Organic InorganicDocument51 paginiOrganic InorganicPatrick GoÎncă nu există evaluări
- H2 Biology - Notes On BiomoleculesDocument9 paginiH2 Biology - Notes On BiomoleculesSefLRho100% (1)
- Chemical Reactions Part II: Week 7: Organic ChemistryDocument24 paginiChemical Reactions Part II: Week 7: Organic Chemistrymia kris quiatchonÎncă nu există evaluări
- Bio Final Review Review Quiz and TestDocument15 paginiBio Final Review Review Quiz and TestLinda ChaoÎncă nu există evaluări
- AP Review: Chapters 2-5Document19 paginiAP Review: Chapters 2-5Min Cheol ShinÎncă nu există evaluări
- Molecular BiologyDocument21 paginiMolecular BiologyvaishnaviÎncă nu există evaluări
- Carbon compounds and organic molecules in biochemistryDocument5 paginiCarbon compounds and organic molecules in biochemistrynaruto710@wuÎncă nu există evaluări
- Lecture 2 - Introductory BiochemistryDocument15 paginiLecture 2 - Introductory BiochemistryJana-Tae KerrÎncă nu există evaluări
- Chemical Composition of The BodyDocument39 paginiChemical Composition of The BodyAyeshaÎncă nu există evaluări
- Biochemistry: Biological MoleculesDocument18 paginiBiochemistry: Biological MoleculesAnup Singh TanwarÎncă nu există evaluări
- Chemistry of Life Elements, Compounds and BondingDocument9 paginiChemistry of Life Elements, Compounds and Bondingraghad mohammedÎncă nu există evaluări
- Chapter 3 The Molecules of CellsDocument5 paginiChapter 3 The Molecules of Cellsmzunl25476Încă nu există evaluări
- Topic I: Introduction To AnatomyDocument3 paginiTopic I: Introduction To AnatomyCristenÎncă nu există evaluări
- General Biology All About BiomoleculesDocument6 paginiGeneral Biology All About BiomoleculeskimberlyfritzzunigaÎncă nu există evaluări
- Biochemistry: Water Carbohydrates Lipids ProteinsDocument16 paginiBiochemistry: Water Carbohydrates Lipids ProteinsryanÎncă nu există evaluări
- Worktext 1 - Biomol - Carbo & Lipids, PERALTADocument10 paginiWorktext 1 - Biomol - Carbo & Lipids, PERALTAKelsi Kyla PeraltaÎncă nu există evaluări
- A Level BiologyDocument522 paginiA Level BiologyJAMESÎncă nu există evaluări
- Biology 1308 Notes on Cell Structure, Energy, and ChemistryDocument21 paginiBiology 1308 Notes on Cell Structure, Energy, and ChemistryAshley CisnerosÎncă nu există evaluări
- Biology 12 Biological Molecules Review KEYDocument5 paginiBiology 12 Biological Molecules Review KEYFeras TarawnehÎncă nu există evaluări
- Molecules of LifeDocument5 paginiMolecules of LifeEmmaÎncă nu există evaluări
- The Chemical Basis of Life Ii: Organic MoleculesDocument47 paginiThe Chemical Basis of Life Ii: Organic Moleculesmeer0091Încă nu există evaluări
- Lecture 4, 5: Chemical Reactions Acids, Bases & SaltsDocument13 paginiLecture 4, 5: Chemical Reactions Acids, Bases & Saltstenzin bhutiÎncă nu există evaluări
- 26 Inorganic and Organic CompoundsDocument6 pagini26 Inorganic and Organic CompoundsMark Allein AguinaldoÎncă nu există evaluări
- Biomolecules (KH, Lemak, Protein) - Topik 3 FBS 1Document47 paginiBiomolecules (KH, Lemak, Protein) - Topik 3 FBS 1Teofilus Dani PÎncă nu există evaluări
- Lectures by Tariq Alalwan, PH.D.: Biology, 12/e Mader & WindelspechtDocument74 paginiLectures by Tariq Alalwan, PH.D.: Biology, 12/e Mader & WindelspechtSulaiman Dawood Saif AbdullaÎncă nu există evaluări
- General Biology - Chap. 03+Document51 paginiGeneral Biology - Chap. 03+정서진Încă nu există evaluări
- Bio Molecules PPT For P AP BiologyDocument34 paginiBio Molecules PPT For P AP BiologyDivineDoctorÎncă nu există evaluări
- Chemical Basis of LifeDocument38 paginiChemical Basis of LifeFernadez RodisonÎncă nu există evaluări
- Biological Molecules: When X y It's Simple So CX (H20) y When X Does Not y It's Simple So CX (H20) yDocument15 paginiBiological Molecules: When X y It's Simple So CX (H20) y When X Does Not y It's Simple So CX (H20) yteeeÎncă nu există evaluări
- Atoms and MoleculesDocument5 paginiAtoms and MoleculesRelaisa CimafrancaÎncă nu există evaluări
- Chapter 4 - Biological MoleculesDocument28 paginiChapter 4 - Biological MoleculesshammmssÎncă nu există evaluări
- Lecture 2 - Introductory BiochemistryDocument17 paginiLecture 2 - Introductory BiochemistryKevonSingh1Încă nu există evaluări
- Biological MoleculesDocument11 paginiBiological MoleculesYasasmi GunasekeraÎncă nu există evaluări
- Week 3 Carbohydrates 2Document26 paginiWeek 3 Carbohydrates 2sabeera.ahmadÎncă nu există evaluări
- Anatomy and Physiology ChemistryDocument49 paginiAnatomy and Physiology ChemistryNoahSullivanÎncă nu există evaluări
- Biology Notes (Lipids)Document5 paginiBiology Notes (Lipids)Teo Jia Ming Nickolas100% (1)
- Biochemistry: Additional Support Materials I.E. Animations, Quizzes, Pictures, WorksheetsDocument26 paginiBiochemistry: Additional Support Materials I.E. Animations, Quizzes, Pictures, WorksheetsnikkikeswaniÎncă nu există evaluări
- Biology As + A2 CombinedDocument253 paginiBiology As + A2 CombinedgalaxyreaderÎncă nu există evaluări
- Notes+4 +ATP,+Water+and+Inorganic+IonsDocument5 paginiNotes+4 +ATP,+Water+and+Inorganic+IonsSyeda Wardah NoorÎncă nu există evaluări
- Molecules of CellsDocument98 paginiMolecules of CellsKd123Încă nu există evaluări
- A-level Biology Revision: Cheeky Revision ShortcutsDe la EverandA-level Biology Revision: Cheeky Revision ShortcutsEvaluare: 5 din 5 stele5/5 (5)
- Anatomy and Physiology For Students: A College Level Study Guide for Life Science and Allied Health MajorsDe la EverandAnatomy and Physiology For Students: A College Level Study Guide for Life Science and Allied Health MajorsÎncă nu există evaluări
- Cell Structure, Processes, and Reproduction, Third EditionDe la EverandCell Structure, Processes, and Reproduction, Third EditionÎncă nu există evaluări
- MCB 7200: Molecular BiologyDocument27 paginiMCB 7200: Molecular BiologytasniyanÎncă nu există evaluări
- IMGDocument6 paginiIMGGeorgiana GenyÎncă nu există evaluări
- MICROBIOLOGY BOARD EXAM QUESTIONSDocument6 paginiMICROBIOLOGY BOARD EXAM QUESTIONSchristinejoan100% (1)
- Non - Green Plants and Insectivorous PlantsDocument21 paginiNon - Green Plants and Insectivorous PlantsJemimaÎncă nu există evaluări
- Analysis of Raw Meats and Fats of Pigs Using Polymerase Chain Reaction (PCR) For Halal AuthenticationDocument6 paginiAnalysis of Raw Meats and Fats of Pigs Using Polymerase Chain Reaction (PCR) For Halal AuthenticationekosaputrobbppbatuÎncă nu există evaluări
- Catch A KillerDocument3 paginiCatch A KillerSavannah QuimbyÎncă nu există evaluări
- 1ST Quarter Test EnviDocument3 pagini1ST Quarter Test EnviFiona Robbie SansanoÎncă nu există evaluări
- Cloning Vectors For EukaryotesDocument14 paginiCloning Vectors For Eukaryotesavbhujle2Încă nu există evaluări
- DNAresonancedraft Max Rempel 2018Document23 paginiDNAresonancedraft Max Rempel 2018Fabrice BresilÎncă nu există evaluări
- LECTURE 2: Continuation Magnification vs. Resolution: Microbio (Midterms Reviewer)Document13 paginiLECTURE 2: Continuation Magnification vs. Resolution: Microbio (Midterms Reviewer)Entris AlcÎncă nu există evaluări
- CTD On BiotechDocument23 paginiCTD On Biotechsandro CardosoÎncă nu există evaluări
- Genotype Phenotype Worksheet PracticeDocument2 paginiGenotype Phenotype Worksheet PracticeIvy NguyenÎncă nu există evaluări
- Steward+ (1955) + +Theory+and+Method+of+Cultural+EcologyDocument14 paginiSteward+ (1955) + +Theory+and+Method+of+Cultural+EcologyAlejandro ArÎncă nu există evaluări
- 1 Part I Central Nervous System 4zaDocument32 pagini1 Part I Central Nervous System 4zaapi-302883249Încă nu există evaluări
- Navy Children School Physical Education Assignment - 1 Class - XiDocument4 paginiNavy Children School Physical Education Assignment - 1 Class - XiToismedÎncă nu există evaluări
- Plant PropagationDocument11 paginiPlant PropagationAbdul Ola IBÎncă nu există evaluări
- Tiger ProjectDocument17 paginiTiger ProjectPooja Bk83% (6)
- A. Monohybrid Crosses: Mendelian Genetics ProblemsDocument4 paginiA. Monohybrid Crosses: Mendelian Genetics ProblemsDoug GilmourÎncă nu există evaluări
- Cellular Transport PowerpointDocument50 paginiCellular Transport PowerpointPortia EgkenÎncă nu există evaluări
- L3.4 AlignmentDocument90 paginiL3.4 AlignmentAzamu Shahiullah ProttoyÎncă nu există evaluări
- Over 40 Scientific BranchesDocument6 paginiOver 40 Scientific BranchesDanÎncă nu există evaluări
- Factors Contributing To Irrational Use of AntibioticsDocument2 paginiFactors Contributing To Irrational Use of AntibioticsgoyeniranÎncă nu există evaluări
- Applied Protozoology and Parasitology Key ConceptsDocument60 paginiApplied Protozoology and Parasitology Key ConceptsIL Mago50% (2)
- Mr. Cruz 11-3 Exploring Mendelian GeneticsDocument21 paginiMr. Cruz 11-3 Exploring Mendelian GeneticsMR. CRUZÎncă nu există evaluări
- Liver Tonic FormulationDocument11 paginiLiver Tonic FormulationMulayam Singh YadavÎncă nu există evaluări
- Practical 2Document6 paginiPractical 2Ah BoonÎncă nu există evaluări
- Molecular and Phylogenetic Analysis of Blastocystis Isolates From Various HostsDocument8 paginiMolecular and Phylogenetic Analysis of Blastocystis Isolates From Various Hostssebasrd07Încă nu există evaluări
- Diabetes Mellitus: The Epidemic of The Century: Akram T Kharroubi, Hisham M DarwishDocument19 paginiDiabetes Mellitus: The Epidemic of The Century: Akram T Kharroubi, Hisham M DarwishAna Cristine Silva Pires TomeÎncă nu există evaluări
- Radiation-Enhanced Stem Cell Differentiation: A Potential New ToolDocument8 paginiRadiation-Enhanced Stem Cell Differentiation: A Potential New TooldwinugrohojuandaÎncă nu există evaluări
- Sardar Patel UniversityDocument1 paginăSardar Patel UniversityPatel BansariÎncă nu există evaluări