Documente Academic
Documente Profesional
Documente Cultură
QA GMP QA Quality Manual
Încărcat de
boimziiDescriere originală:
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
QA GMP QA Quality Manual
Încărcat de
boimziiDrepturi de autor:
Formate disponibile
Radiopharmaceutical Production
Quality Manual
STOP
Quality Manual
A Quality Manual documents the policies,
procedures, responsibilities, and
documentation, that must be in place
for the facility to comply with their
regulatory responsibilities.
Contents
Purpose of the Quality
Manual
Contents of the Quality
Manual
Relationship of the Quality
Manual to the Validation
Master Plan
Examples of Quality Manuals
STOP
Radiopharmaceutical
Production
Quality Manual
Contents
Purpose of the Quality
Manual
Contents of the Quality
Manual
Relationship of the
Quality Manual to the
Validation Master Plan
Examples of Quality
Manuals
STOP
Purpose of the Quality
Manual
The organization should establish and maintain a quality manual
that includes
a) the quality policy
b) the scope of the quality management system, including
details of and justification for any exclusions
c) the documented procedures established for the quality
management system, or reference to them, and
d) a description of the interaction between the processes of the
quality management system.
Radiopharmaceutical
Production
Quality Manual
Contents
Purpose of the Quality
Manual
Contents of the Quality
Manual
Relationship of the
Quality Manual to the
Validation Master Plan
Examples of Quality
Manuals
STOP
Contents of the Quality
Manual
A quality manual is a document that a facility writes to explain
which regulations are applicable to the facility (i.e. which
regulations will be followed by the facility.)
The regulations that are to be followed are based on the
process(es) that are performed at the facility.
The facilitys Quality Manual should outline which regulations
are going to be followed, how they are going to be followed,
who is responsible for ensuring that the regulations are
followed, and which of the companies approved procedures
address the regulations to be followed. If all parts of a regulation
are not going to be followed, a facility may want to include a
brief explanation as to why a part of a regulation is not
applicable to the facility and therefore will not be outlined in the
Quality Manual.
The Quality Manual should be written in general terms with
minimal specifics. The format of a Quality Manual is usually
different than the format used for the facilitys other approved
documents. The format should still include such things as a
facilitys name, version control, and approval signatures.
Radiopharmaceutical
Production
Quality Manual
Contents
Purpose of the Quality
Manual
Contents of the Quality
Manual
Relationship of the
Quality Manual to the
Validation Master Plan
Examples of Quality
Manuals
STOP
Contents of the Quality
Manual
The following are examples of sections that a Quality Manual
should contain:
Table of Contents
Introduction
Facility Background
Purpose
Scope
Quality Policies and Objectives
Organization and Structure of Documentation
Facilitys Products
References
Quality Policies for Specific Regulation Elements
Table of Contents: A list of the sections contained within the
Quality Manuel and the page each section begins on.
Introduction: A brief description of the facility, the facilitys
purpose for writing a Quality Manual, and a brief description of
the scope of the Quality Manual.
Radiopharmaceutical
Production
Quality Manual
Contents
Purpose of the Quality
Manual
Contents of the Quality
Manual
Relationship of the
Quality Manual to the
Validation Master Plan
Examples of Quality
Manuals
STOP
Contents of the Quality
Manual
Background: List the name of the facility, where the facility is
located, what radiopharmaceutical products are produced at the
facility, and how these will be distributed.
Purpose: Provide general statements explaining why and how
the Quality Manual will be used.
Example: This Quality Manual describes the quality management
system that has been established by facility X in order to meet
regulatory requirements for the production of PET
radiopharmaceuticals in the United State.
Scope: List the regulations that will be followed by the facility as
well as any portions of the regulations that will not be followed.
Provide a justification for why those portions of the regulations
are not going to be followed.
Example: The system described in this manual is intended to meet
general requirements set forth in the United States Food and Drug
Administration (FDA) Regulation according to 21CFR Part 210,
21CFR Part 211 and 21CFR Part 212 as well as the relevant
section of 21CFR Part 823.
Radiopharmaceutical
Production
Quality Manual
Contents
Purpose of the Quality
Manual
Contents of the Quality
Manual
Relationship of the
Quality Manual to the
Validation Master Plan
Examples of Quality
Manuals
STOP
Contents of the Quality
Manual
Quality Policies and Objectives: Include a brief statement about
the approach that the facility is taking in regards to quality, (i.e.
a Quality Mission Statement) and list the quality objectives of
the facility. The quality objectives should not be numerous (five
to seven is a typical number) and should be briefly stated.
Example: Customer issues are addressed in a timely, professional,
and thorough manner.
Example: Personnel are adequately trained in the job they perform
Example: Products and Services provided by our facility are
designed, manufactured, and delivered to our customers and meet
or exceed our customer requirements.
Organization and Structure of Documentation: Provide an
explanation as to how the documentation structure at your
facility is organized and managed in relation to the applicable
regulations.
Example: Documents related to the quality system are organized in
a hierarchy structure, are maintained in accordance with applicable
regulatory requirements and our facility record retention policy, and
are managed through a document change control system.
Radiopharmaceutical
Production
Quality Manual
Contents
Purpose of the Quality
Manual
Contents of the Quality
Manual
Relationship of the
Quality Manual to the
Validation Master Plan
Examples of Quality
Manuals
STOP
Contents of the Quality
Manual
Products: This section should include a brief description of the
products that are made and distributed by the facility including
what the intended use of the products is to be. This description
should be similar to a marketing type summary in that it does
not list proprietary or explicit product information that would be
detrimental to the facility if persons outside the facility read the
description. This section should provide enough detail to justify
the sections of the regulations that are and are not going to be
followed.
References: A list of all the different regulations that were sited
within the Quality Manual.
Example: FDA Title 21, Code of Federal Regulations Part 210 &
211, Current Good Manufacturing Practice in Manufacturing,
Processing, Packing, or Holding of Drugs and Finished
Pharmaceuticals, FDA Title 21, Code of Federal Regulations Part
212 PET Drugs Current Good Manufacturing Practice (cGMP),
Radiopharmaceutical
Production
Quality Manual
Contents
Purpose of the Quality
Manual
Contents of the Quality
Manual
Relationship of the
Quality Manual to the
Validation Master Plan
Examples of Quality
Manuals
STOP
Contents of the Quality Manual
Quality Policies for Specific Regulation Elements: This section will
encompass the majority of the manual. It will include each
regulation that will be followed by the facility, a brief description of
how the facility intends to follow the regulation, and a list of the
approved documents (by document name and number) that the
facility has in place which specifically address/discuss the facilitys
policies and objectives as stated in this section of the Quality
Manual.
Example: Purchasing Controls; Procedures are established and
maintained to ensure that materials, supplies, and services impacting
the Quality System and procured by facility X purchasing department,
conform to specified requirements.
Receiving Procedure SOP # XX. 01 Vendor Qualification Procedure SOP #
XX. 01 Release of In-coming Goods Procedure SOP # XX. 01 ......
Example: Corrective and Preventive Action; At facility X, Corrective
and Preventive Actions are integral in a variety of programs.
Whenever possible, these programs make an effort to determine the
root cause of the incident and to implement appropriate actions to
prevent the reoccurrence of the incident. Quality Assurance approves
all Corrective and Preventive plans and tracks their completion and
effectiveness.
CAPA Procedure SOP # XX. 01 Material Review Board SOP # XX. 01
Failure Investigation Procedure SOP # XX. 01 .......
Radiopharmaceutical
Production
Quality Manual
Contents
Purpose of the Quality
Manual
Contents of the Quality
Manual
Relationship of the
Quality Manual to the
Validation Master Plan
Examples of Quality
Manuals
STOP
Contents of the Quality Manual
Deviations and Events
Event Recording
Reviews and
Investigations
Remediation
Change Control
Change Initiation and
Approval
Change Execution and
Tracking
Change Verification and
Closure
Audit Management
Planning
Execution
Review and Remediation
Document Management
Storing and
Classification
Access and Viewing
Creating New
Documents
Lifecycle Managements
Printing
Training Management
Employee Course
Management
Training Request and
Approval
Gap Management and
Records
Equipment Management
Inventory Management
Preventative Maintenance
Remedial Maintenance
Topics that should be covered in the final section on
Quality Policies for Specific Regulation Elements
Radiopharmaceutical
Production
Quality Manual
Contents
Purpose of the Quality
Manual
Contents of the Quality
Manual
Relationship of the
Quality Manual to the
Validation Master Plan
Examples of Quality
Manuals
STOP
Contents of the Quality Manual
General Requirements
Security and Access Control
Facility Management
Corrective Action Preventive
Action (CAPA)
CAPA Initiation
Investigations and Action
Plan
CAPA Closure
Validation
Documentation Structure
and Formats
Change Control
Planning and Scheduling
Roles and Responsibilities
Regulatory Requirements
for Testing
A list of SOPs, Forms,
Validation Documentation
and other appendicies
Topics that should be covered in the final section on
Quality Policies for Specific Regulation Elements
Radiopharmaceutical
Production
Quality Manual
Contents
Purpose of the Quality
Manual
Contents of the Quality
Manual
Relationship of the
Quality Manual to the
Validation Master Plan
Examples of Quality
Manuals
STOP
Relationship of the Quality Manual
to the Validation Master Plan
Quality Manual: The quality manual is a document that a
facility writes to explain which portions of which regulations are
applicable to the facility and which documents the policies,
procedures, responsibilities, and documentation, that must be in
place for the facility to comply with these regulatory
responsibilities
Validation Master Plan: The Validation Master Plan is a
summary plan which communicates managements
expectations and commitments to be followed for the sites
validation program including the responsibilities and is therefore
a key document at a site. It describes the program to be
conducted to get the items in question in a validated manner.
The plan lists all of the validation activities to be completed, as
well as the schedule for their completion. The term validation is
used to demonstrate with written evidence that the item under
consideration, e.g. process does what it purports to do.
Validation includes but is not limited to: equipment, computer
systems, production processes, cleaning procedures, facilities,
utilities as well as analytical methods.
Radiopharmaceutical
Production
Quality Manual
Contents
Purpose of the Quality
Manual
Contents of the Quality
Manual
Relationship of the
Quality Manual to the
Validation Master Plan
Examples of Quality
Manuals
STOP
Example of a Quality Manual
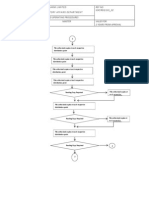
One approach to the Quality Manual is to delineate the quality
policies for specific regulation elements by making a flow chart
of every process and then assigning the SOP or other
document which addresses that process to ensure that
everything is covered with an SOP. An example of this is shown
on the next slide.
Note that each process or activity has an SOP number or
document associated with it.
The SOP numbers starting with A are the administrative SOPs
while those starting with Q are the quality assurance SOPs.
Radiopharmaceutical
Production
Quality Manual
Contents
Purpose of the Quality
Manual
Contents of the Quality
Manual
Relationship of the
Quality Manual to the
Validation Master Plan
Examples of Quality
Manuals
STOP
Example of a Quality Manual
QA Unit
Post-Release
Testing
Personnel
Equipment
Control
Production
Process Control
Pre-Release
Testing
Batch Record
Facility Control
Document Control
Equipment
Qualification
Equipment Log Book
Review
Annual Document Review
Equipment Log
Books
Anomalies/
Deviations
Pre-Release
A008 A007
Master Equipment List
Review
Reagent and Solvent Log
Book Review
Internal Review
Waste Disposal
Employee Job
Training File
[
11
C] GMP
Process
Post-Release
Component Inventory
Review
Name/Signature Form
Q023
Q034
Non-Conformance Report
Q025
Employee Job
Training File
Security
Pest Control
Q037
Q028
Q029
QS001
BNL Contracts
O_M Procedures
Preparation
Quality Policy
Red Hot Cell Diagram, D001 and
Red Hot Cell Process, D002
Q021, C020, Q005, Q038, Q004,
Q002, Q010, Q013, Q027
Production Framework
Diagram, D004
Production Framework
Diagram, D004
Pre- and Post-Release Testing
Diagram, D004
Pre- and Post-Release Testing
Diagram, D004
Q022
CA001-CA005
W001
Package and
Shipping
A005
Specific Activity Q015
Release
Q033
Training Matrix
Master Equipment List
Master Document List
Internal Audit Form
Name/Signature Form
CAPA Form Problem Reporting/CA
Q002, Q011, Q013, Q018, Q020,
Q021Q026, Q031, Q032, Q034,
Q035, Q044,
Radiopharmaceutical
Production
Quality Manual
Contents
Purpose of the Quality
Manual
Contents of the Quality
Manual
Relationship of the
Quality Manual to the
Validation Master Plan
Examples of Quality
Manuals
STOP
Example of a Quality Manual
Another example of a Quality Manual with less detail but
covering the major elements can be found by following the
arrow.
Quality Manual Example More
Return to Main Menu
S-ar putea să vă placă și
- Good Distribution Practice A Complete Guide - 2021 EditionDe la EverandGood Distribution Practice A Complete Guide - 2021 EditionÎncă nu există evaluări
- Validation Master Plan A Complete Guide - 2021 EditionDe la EverandValidation Master Plan A Complete Guide - 2021 EditionÎncă nu există evaluări
- How To Build Up A GMP Quality ManualDocument17 paginiHow To Build Up A GMP Quality Manualjessari7396586% (7)
- SOP065.3 - 01 Raw Materials and Development of Spec For Cat ODocument7 paginiSOP065.3 - 01 Raw Materials and Development of Spec For Cat OGrace Chen100% (1)
- Quality Manual TemplateDocument34 paginiQuality Manual Templatesakib445100% (3)
- Q Pharma Quality ManualDocument32 paginiQ Pharma Quality Manualsappz354544883% (6)
- Product RecallDocument18 paginiProduct Recallmarkandey guptaÎncă nu există evaluări
- Toc GMP Manual Ud12Document34 paginiToc GMP Manual Ud12navas1972100% (2)
- Line Clearance ProcedureDocument3 paginiLine Clearance ProcedurePrince Moni67% (3)
- Quality ManualDocument16 paginiQuality ManualMichael Moore100% (4)
- Product Quality Review FlowDocument1 paginăProduct Quality Review Flownasreen anjumÎncă nu există evaluări
- GMP Audit Checklist For ManufacturersDocument90 paginiGMP Audit Checklist For ManufacturersJagtar Singh Chandel100% (2)
- 007 Out of SpecificationDocument12 pagini007 Out of Specificationmarkandey gupta100% (2)
- GMP Quality Assurance ProceduresDocument22 paginiGMP Quality Assurance ProceduresLen Surban100% (1)
- Development of CCSDocument54 paginiDevelopment of CCSDiana Oldani100% (2)
- SOP For Qualification of Vendors - Pharmaceutical GuidelinesDocument3 paginiSOP For Qualification of Vendors - Pharmaceutical Guidelineskavya nainita100% (1)
- Manufacturing Practice Audit: (GMP Audit - Check List)Document31 paginiManufacturing Practice Audit: (GMP Audit - Check List)Sandra100% (1)
- Validation VMP Validation Master PlanDocument13 paginiValidation VMP Validation Master Plank.p.Încă nu există evaluări
- Guideline Supplier Qualification - Contract Labs Final - Jan 2012Document22 paginiGuideline Supplier Qualification - Contract Labs Final - Jan 2012nsk79in@gmail.com100% (1)
- QRM SOP Issue# 01 ApprovedDocument9 paginiQRM SOP Issue# 01 ApprovedibrahimgomaaÎncă nu există evaluări
- Batch Release For Goods: 1. ObjectiveDocument2 paginiBatch Release For Goods: 1. ObjectivePrince MoniÎncă nu există evaluări
- ReprocessingDocument3 paginiReprocessingswanandkul86% (7)
- 9 C Validation Protocol TABLETDocument20 pagini9 C Validation Protocol TABLETMohammed ZubairÎncă nu există evaluări
- Validation Master Plan TemplateDocument17 paginiValidation Master Plan TemplateNadine100% (4)
- Sop For Handling of Returned Goods: II. Scope Iii. ResponsibilityDocument2 paginiSop For Handling of Returned Goods: II. Scope Iii. Responsibilitysachin100% (4)
- GMP Audit ChecklistDocument36 paginiGMP Audit Checklistshahzadagp80% (5)
- Site Master File: OF Solitaire Pharmacia PVT - LTDDocument35 paginiSite Master File: OF Solitaire Pharmacia PVT - LTDsolitairepharmacia50% (2)
- Validation PolicyDocument3 paginiValidation PolicyneppoanandÎncă nu există evaluări
- 033 - SOP On Batch Release SystemDocument3 pagini033 - SOP On Batch Release SystemDevender Malhotra86% (22)
- Change Control Manufacturing MatrixDocument4 paginiChange Control Manufacturing MatrixPrem GoelÎncă nu există evaluări
- SOP Equipment ValidationDocument15 paginiSOP Equipment Validationfarjana100% (7)
- Procedure For Cleaning of Production AreaDocument3 paginiProcedure For Cleaning of Production Areaasit_m50% (6)
- Eu GMPDocument16 paginiEu GMPamirin_kingÎncă nu există evaluări
- Sop For Annual Product ReviewDocument1 paginăSop For Annual Product ReviewPrince Moni100% (1)
- GMP Supplier Assessment QuestionnaireDocument2 paginiGMP Supplier Assessment Questionnairedrs_mdu48Încă nu există evaluări
- Annual Product Review Developing An SOPDocument26 paginiAnnual Product Review Developing An SOPanants2567% (3)
- ISPE ChecklistDocument6 paginiISPE Checklistm_ihab777629Încă nu există evaluări
- Lifecycle Approach To Cleaning ValidationDocument60 paginiLifecycle Approach To Cleaning Validational68130Încă nu există evaluări
- Site Master FileDocument66 paginiSite Master Filekannanppharma100% (3)
- Site Master FileDocument6 paginiSite Master FileRambabu komati - QA100% (3)
- SOP For Handling of Market Complaints in Pharmaceuticals - Pharmaceutical GuidelinesDocument6 paginiSOP For Handling of Market Complaints in Pharmaceuticals - Pharmaceutical Guidelinesmoinpharm100% (1)
- QualityAgreementGuide2009 (Issued) FinalDocument24 paginiQualityAgreementGuide2009 (Issued) Finaldrs_mdu48100% (1)
- QMSSOP049 - 01 Supplier QualificationDocument13 paginiQMSSOP049 - 01 Supplier QualificationMohamed Kamal100% (1)
- Quality Manual FinalDocument19 paginiQuality Manual FinalFarhan TaseenÎncă nu există evaluări
- 014 Quality Unit Roles and ResponsibilitiesDocument35 pagini014 Quality Unit Roles and ResponsibilitiesSIRAJ KP100% (1)
- SOP For Quality Risk Management - Pharmaceutical GuidelinesDocument3 paginiSOP For Quality Risk Management - Pharmaceutical Guidelinessakib44586% (7)
- Audit Checklist SOPDocument43 paginiAudit Checklist SOPthemba100% (4)
- GMP MANUAL Contents: 1 Pharmaceutical Quality System (PQS)Document41 paginiGMP MANUAL Contents: 1 Pharmaceutical Quality System (PQS)M. S. Chikkamani100% (1)
- Laboratory Quality Agreement TamplateDocument10 paginiLaboratory Quality Agreement TamplateMina Maher MikhailÎncă nu există evaluări
- List of SOP's For Quality Assurance DepartmentDocument1 paginăList of SOP's For Quality Assurance DepartmentPrince Moni100% (1)
- Sop QualificationDocument9 paginiSop Qualificationjohn100% (1)
- SOP On Product Recall - Pharmaceutical GuidanceDocument5 paginiSOP On Product Recall - Pharmaceutical GuidanceGanesh Kashinath100% (2)
- Unplanned Cleanroom Power Outage Time Limit and Recovery Determinations For Aseptic Processing AreasDocument2 paginiUnplanned Cleanroom Power Outage Time Limit and Recovery Determinations For Aseptic Processing Areasananth100% (1)
- SOP For Self Inspection and Internal Audits - Pharmaceutical GuidelinesDocument2 paginiSOP For Self Inspection and Internal Audits - Pharmaceutical GuidelinesJose Ramon Dalo Bautista100% (1)
- Concepts of Quality Management in Pharmaceutical IndustryDe la EverandConcepts of Quality Management in Pharmaceutical IndustryÎncă nu există evaluări
- Standard Operating Procedure For QPPV Role and ResponsibilitiesDocument7 paginiStandard Operating Procedure For QPPV Role and Responsibilitiesboimzii100% (1)
- Standard Operating Procedure For Regulatory InteligenceDocument5 paginiStandard Operating Procedure For Regulatory Inteligenceboimzii100% (2)
- Standard Operating Procedure For Signal DetectionDocument9 paginiStandard Operating Procedure For Signal Detectionboimzii100% (1)
- Regulatory Intelligence FormDocument1 paginăRegulatory Intelligence FormboimziiÎncă nu există evaluări
- Standard Operating Procedure For Key Peformance IndicatorsDocument8 paginiStandard Operating Procedure For Key Peformance Indicatorsboimzii100% (2)
- Standard Operating Procedure For Literature SerchesDocument8 paginiStandard Operating Procedure For Literature SerchesboimziiÎncă nu există evaluări
- Databases and Product List Reconciliation FormDocument1 paginăDatabases and Product List Reconciliation FormboimziiÎncă nu există evaluări
- Master Soppd101 02Document23 paginiMaster Soppd101 02boimziiÎncă nu există evaluări
- Final Copy of The Master SOP PDFDocument1 paginăFinal Copy of The Master SOP PDFboimziiÎncă nu există evaluări
- Final Eac Guidelines For Preparation of A Site Master FileDocument17 paginiFinal Eac Guidelines For Preparation of A Site Master FileboimziiÎncă nu există evaluări
- Oracle Fusion Global Human Resources Payroll Costing GuideDocument90 paginiOracle Fusion Global Human Resources Payroll Costing GuideoracleappshrmsÎncă nu există evaluări
- Garvida V SalesDocument1 paginăGarvida V SalesgemmaÎncă nu există evaluări
- Notification: Professional Examination Result: November-December 2020Document2 paginiNotification: Professional Examination Result: November-December 2020Zahidul Amin FarhadÎncă nu există evaluări
- Music Tradition of Kamrup-KamakhyaDocument15 paginiMusic Tradition of Kamrup-KamakhyaDilip ChangkakotyÎncă nu există evaluări
- Kishore M. Daswani - ResumeDocument3 paginiKishore M. Daswani - ResumeKishore DaswaniÎncă nu există evaluări
- Wallem Philippines Shipping vs. SR Farms (2010)Document1 paginăWallem Philippines Shipping vs. SR Farms (2010)Teff QuibodÎncă nu există evaluări
- Rizal ParaniDocument201 paginiRizal ParaniMuhammadd YaqoobÎncă nu există evaluări
- BUSINESS PLAN NON-DISCLOSURE AGREEMENT Sameer PatelDocument4 paginiBUSINESS PLAN NON-DISCLOSURE AGREEMENT Sameer Patelsameer9.patelÎncă nu există evaluări
- The Role of Women in Trade Unions and Nation BuildingDocument18 paginiThe Role of Women in Trade Unions and Nation BuildingSneha KanitCar Kango100% (1)
- Theological Seminary of Evengelical Kalimantan ChurchDocument15 paginiTheological Seminary of Evengelical Kalimantan ChurchPetra Aria Pendung Wu'iÎncă nu există evaluări
- Rizal FamilyDocument3 paginiRizal FamilyPamela MarquezÎncă nu există evaluări
- Priyanshu Mts Answer KeyDocument34 paginiPriyanshu Mts Answer KeyAnima BalÎncă nu există evaluări
- 8299 PDF EngDocument45 pagini8299 PDF Engandrea carolina suarez munevarÎncă nu există evaluări
- Star Wars Canon Timeline MythBankDocument25 paginiStar Wars Canon Timeline MythBankJonathan Carson100% (1)
- Aims of The Big Three'Document10 paginiAims of The Big Three'SafaÎncă nu există evaluări
- Business Finance: Quarter 2 - Module 6: Philosophy and Practices in Personal FinanceDocument2 paginiBusiness Finance: Quarter 2 - Module 6: Philosophy and Practices in Personal FinanceClemente AbinesÎncă nu există evaluări
- 10 Types of Innovation (Updated)Document4 pagini10 Types of Innovation (Updated)Nur AprinaÎncă nu există evaluări
- Mt-Requirement-01 - Feu CalendarDocument18 paginiMt-Requirement-01 - Feu CalendarGreen ArcÎncă nu există evaluări
- Pidato Bhs Inggris The HomelandDocument1 paginăPidato Bhs Inggris The HomelandYeni NuraeniÎncă nu există evaluări
- GCTADocument5 paginiGCTAPatty CabañaÎncă nu există evaluări
- 202E13Document28 pagini202E13Ashish BhallaÎncă nu există evaluări
- Youtube/Ydsatak Tense Ders 1Document9 paginiYoutube/Ydsatak Tense Ders 1ArasIlgazÎncă nu există evaluări
- Islam and PatriarchyDocument21 paginiIslam and PatriarchycarolinasclifosÎncă nu există evaluări
- Balkan Nationalism (Shodhangana Chapter) PDFDocument40 paginiBalkan Nationalism (Shodhangana Chapter) PDFsmrithiÎncă nu există evaluări
- GS Mains PYQ Compilation 2013-19Document159 paginiGS Mains PYQ Compilation 2013-19Xman ManÎncă nu există evaluări
- Blcok 5 MCO 7 Unit 2Document12 paginiBlcok 5 MCO 7 Unit 2Tushar SharmaÎncă nu există evaluări
- Nurlilis (Tgs. Bhs - Inggris. Chapter 4)Document5 paginiNurlilis (Tgs. Bhs - Inggris. Chapter 4)Latifa Hanafi100% (1)
- Biopolitics and The Spectacle in Classic Hollywood CinemaDocument19 paginiBiopolitics and The Spectacle in Classic Hollywood CinemaAnastasiia SoloveiÎncă nu există evaluări
- Dawah Course Syllabus - NDocument7 paginiDawah Course Syllabus - NMahmudul AminÎncă nu există evaluări
- L1 Support-Windows Server Interview Question & AnswersDocument6 paginiL1 Support-Windows Server Interview Question & AnswersSmile Ever100% (3)