Documente Academic
Documente Profesional
Documente Cultură
Evaporation: DKK3433 Unit Operation Dr. Syed Mohd Saufi 2012/2013-I
Încărcat de
AzmiHafifiDescriere originală:
Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Evaporation: DKK3433 Unit Operation Dr. Syed Mohd Saufi 2012/2013-I
Încărcat de
AzmiHafifiDrepturi de autor:
Formate disponibile
+
DKK3433
UNIT OPERATION
Dr. Syed Mohd Saufi
2012/2013-I
1
Evaporation
+
Falling Film Type Evaporator
Dr SMS 2012/2013
2
http://www.youtube.com/watch?v=3T8Km9BYHeg&playnext=1&list=PL4ECF5DA8511498E6&feature=results_main
+
3
Contents
Introduction
Processing Factor in Evaporation
Type of Evaporation
Method of Operation of Evaporators
Calculation Method for Single Effect Evaporator
Calculation Method for Multiple Effect Evaporator
+
4
Introduction
Evaporation is achieved by adding heat to the solution to
vaporize the solvent.
Vapor (usually water) from a boiling liquid solution is removed
and a more concentrated solution remains.
Heat is provided by the condensation of a vapor (such as
steam) on one side of a metal surface with the evaporating
liquid on the other side
The normal heating medium is low pressure exhaust steam
from turbines, special heat transfer fluids or flue gases.
Example: concentration of aqueous solutions of sugar, sodium
chloride, glue, milk and orange juice.
In some case, the purpose of evaporation is to concentrate the
solution so that upon cooling, salt crystal will be formed and
separate
+
Basic Operation of Evaporator
The typical evaporator is made up of three functional
sections: the heat exchanger, the evaporating section,
where the liquid boils and evaporates, and the
separator in which the vapour leaves the liquid and
passes off to the condenser or to other equipment.
In many evaporators, all three sections are contained
in a single vertical cylinder.
In the center of the cylinder there is a steam heating
section, with pipes passing through it in which the
evaporating liquors rise.
At the top of the cylinder, there are baffles, which allow
the vapours to escape but check liquid droplets that
may accompany the vapours from the liquid surface.
In the heat exchanger section, called a calandria in this
type of evaporator, steam condenses in the outer
jacket and the liquid being evaporated boils on the
inside of the tubes and in the space above the upper
tube plate.
The resistance to heat flow is imposed by the steam
and liquid film coefficients and by the material of the
tube walls.
Dr SMS 2012/2013
5
http://www.nzifst.org.nz/unitoperations/evaporation1.htm
+
Basic Operation of Evaporator
The circulation of the liquid greatly affects evaporation rates, but
circulation rates and patterns are very difficult to predict in any detail.
With dissolved solids in increasing quantities as evaporation proceeds
leading to increased viscosity and poorer circulation, heat transfer
coefficients in practice may be much lower than this.
As evaporation proceeds, the remaining liquors become more
concentrated and because of this the boiling temperatures rise. The rise in
the temperature of boiling reduces the available temperature drop,
assuming no change in the heat source. And so the total rate of heat
transfer will drop accordingly.
Also, with increasing solute concentration, the viscosity of the liquid will
increase, often quite substantially, and this affects circulation and the heat
transfer coefficients leading again to lower rates of boiling.
Yet another complication is that measured, overall, heat transfer
coefficients have been found to vary with the actual temperature drop, so
that the design of an evaporator on theoretical grounds is inevitably
subject to wide margins of uncertainty.
Dr SMS 2012/2013
6
http://www.nzifst.org.nz/unitoperations/evaporation1.htm
+
Processing Factor in Evaporation
7
1. Concentration in liquid
2. Solubility
3. Temperature sensitivity of materials
4. Foaming or frothing
5. Pressure and temperature
6. Scale deposition and materials of construction
+
8
1. Concentration in liquid
Usually liquid feed to evaporation is relatively dilute and has a lower
viscosity and higher heat transfer coefficient, h
As evaporation proceeds, the solution become more concentrate and
high viscosity, then will drop the heat transfer coefficient value.
Therefore, adequate circulation and turbulence must be present to keep
the h value becoming too low.
2. Solubility
As solutions are heated, the concentration of solute increase and
solubility is decrease and can be exceed the solubility limit of the
solution, then the crystal formed.
Solubility is increase as temperature increase. This means when hot
concentrated solution from evaporation is cooled to room temperature,
crystallization may occur.
3. Temperature sensitivity of materials
Many food products or biological materials may be temperature sensitive
and degrade at higher temperatures or after prolonged heating.
Must be considered in the operation of evaporation.
Processing Factor in Evaporation
+
9
Processing Factor in Evaporation
4. Foaming and frothing
Caustic solutions, some food solutions such as milk, some fatty acid
solutions form foam/froth during boiling.
This foam will losses from the solution by the vapor comes out from the
evaporation.
5. Pressure and temperature
Higher operating pressure, higher boiling temperature of the solution
As concentration of the solution increased by evaporation, the
temperature of boiling may rise- called boiling point rise (BPR)
To keep the temperatures low in heat sensitive materials, it is often
necessary to operate under 1 atm (i.e under vacuum)
6. Scale deposition and materials of construction
Some solid material can be deposit on the heating surface of the
evaporation, this will reduce the overall heat transfer coefficient and
cleaning is necessary.
Material for construction of evaporation must be minimize corrosion
phenomena.
+
Rate of Evaporation
The basic factors that affect the rate of evaporation are the:
rate at which heat can be transferred to the liquid
quantity of heat required to evaporate each kg of water
maximum allowable temperature of the liquid
pressure at which the evaporation takes place
changes that may occur in the foodstuff during the course of the
evaporation process.
Important practical considerations in evaporators are the:
maximum allowable temperature, which may be substantially below
100C.
promotion of circulation of the liquid across the heat transfer surfaces,
to attain reasonably high heat transfer coefficients and to prevent any
local overheating,
viscosity of the fluid which will often increase substantially as the
concentration of the dissolved materials increases,
tendency to foam which makes separation of liquid and vapour difficult.
Dr SMS 2012/2013
10
http://www.nzifst.org.nz/unitoperations/evaporation1.htm
+
Type of Evaporator
Open kettle or pan
Horizontal-tube natural circulation evaporator
Vertical-type natural circulation evaporator
Long-tube vertical-type evaporator
Falling-film type evaporator
Forced-circulation-type evaporator
Agitated-film evaporator
Open-pan solar evaporator
11
+
Open Kettle/Pan Evaporator
heat is supplied by
condensation od steam in a
jacket or in coils immersed in
the liquid
in some cases, kettle is direct
fired
inexpensive and simple to use
heat economy is poor
in some cases, paddles or
scrapers are used for agitation
Dr SMS 2012/2013
12
9/ 12/ 12 12:07 PM
Page 1 of 1 f i l e:/ / / Users/ smsauf i / Document s/ 00%20Sugay%20Sync/ Akademik/ 2012- 2013- I/ Uni t %20Operat i on/ Evaporat or2.swf
http://rpaulsingh.com/animated%20figures/fig8_4.htm
+
Horizontal Tube Natural Circulation Evaporator
The horizontal bundle of heating tubes
similar to heat exchanger is used
The steam enters the tubes, where it
condenses, leaves at the other end of the
tubes.
The boiling liquid solution covers the tubes.
The vapor leaves the liquid surface, often
goes through some de-entraining device
such as baffle to prevent carryover of liquid
droplets, and leaves out the top.
Relatively cheap, used for nonviscous
liquids with high heat-transfer coefficient
and liquid that do not deposit scale.
Dr SMS 2012/2013
13
+
Vertical Type Natural Circulation Evaporator
The liquid is inside the tubes and
the steam condenses outside the
tubes
Because of boiling and decreases
in density, the liquid rises in the
tubes by natural circulation, and
flows downward through a large,
central open space or down
comer.
Often called as short-tube
evaporator
Dr SMS 2012/2013
14
+
Long Tube Vertical Type Evaporator
The tubes are 3 to 10 m long
and the formation of vapor
bubbles inside the tubes
causes a pumping action,
which gives quite high liquid
velocities
Liquid passes through the
tubes only once and is not
recirculates. Contact time can
be quite low in this type of
evaporator.
In some cases, as when the
ratio of feed to evaporation
rate is low, recirculation is
made by adding large pipe
connection between the
outlet concentrate line and
the feed line
Dr SMS 2012/2013
15
http://rpaulsingh.com/animated%20figures/fig8_5.htm
http://rpaulsingh.com/animated%20figures/fig8_6.htm
+
Falling Film Type Evaporator
Liquid is fed to the top of the tubes and flows down the walls as thin film
V-L separation take place at the bottom
widely used for concentrating heat sensitive materials such as fruit juices
Dr SMS 2012/2013
16
http://rpaulsingh.com/animated%20figures/fig8_7.htm
http://www.niroinc.com/evaporators_crystallizers/fa
lling_film_evaporators.asp
+
Falling Film Type Evaporator
Dr SMS 2012/2013
17
http://www.youtube.com/watch?v=3T8Km9BYHeg&playnext=1&list=PL4ECF5DA8511498E6&feature=results_main
+
Forced Circulation Type Evaporator
Used pump to circulate the liquid
Increase liquid-film heat transfer
Use for viscous liquids
Dr SMS 2012/2013
18
http://www.niroinc.com/evaporators_crystallizers/forced
_circulation_evaporator.asp
+
Forced Circulation Type Evaporator
Dr SMS 2012/2013
19
http://www.youtube.com/watch?feature=endscreen&list=PL4ECF5DA8511498E6&NR=1&v=22W753joAnA
+
Agitated Film Evaporator
Mechanical agitation of liquid
film to increase turbulence in
this film, and hence the heat
transfer coefficient
Modification of falling film
evaporator with only a single ,
large, jacketed tube containing
an internal agitator.
Liquid enters at the top of the
tube and as it flows downward,
it is spread out into a turbulent
film by vertical agitator blades.
The concentrated solution
leaves at the bottom and vapor
leaves through a separator and
out the top.
Dr SMS 2012/2013
20
http://www.technoforce.net/agitate
d-thin-film-evaporators.html
http://distilleryplants.tradeindia.com/agita
ted-thin-film-evaporator-355261.html
+
Method of Operation of Evaporators
Single effect evaporators
Forward feed multiple effect evaporators
Backward feed multiple effects evaporators
Parallel feed multiple effect evaporators
Dr SMS 2012/2013
21
+
Single Effect Evaporators
The solution in the evaporator is assumed to be completely mixed, the concentrated
product and the solution in evaporator have the same composition and temperature T
1
,
which is the boiling point of solution at P
1
.
The temperature of the vapor is also at T
1
, since it is equilibrium with the boiling solution.
The pressure is P
1
, which is the vapor pressure of the solution at T
1
.
Often used when the required capacity of operation is relatively small and the cost of
steam is relatively cheap compared to the evaporator cost
However, energy utilization is poor since the latent heat of the vapor leaving is not used
but is discarded.
Dr SMS 2012/2013
22
Feed, F
T
F
, x
F
, h
F
.
Steam, S
T
S
, H
S
Concentrated liquid, L
T
1
, x
L
, h
L
Condensate, S
T
S
, h
S
Vapor, V
T
1
, y
V
, H
V
P
1
T
1
heat-exchanger
tubes
to condenser
The rate of heat transfer (q : W, btu/h)
U : overall heat transfer coefficient, W/m
2
.K;
btu/h.ft
2
.F
A : heat transfer area, m
2
; ft
2
T
s
, T
1
: in K; F
Ts is temperature of condensing steam
q =UA T =UA(T
s
T
1
)
+
Forward Feed Multiple Effect Evaporators
The fresh feed is added to the first effect and flows to the next in the same
direction as the vapor flow.
Used when the feed hot or when the final concentrated product might be
damaged at high temperature.
At steady-state operation, the flow rates and the rate of evaporation in each
effect are constant.
The boiling temperature decrease from effect to effect, cause pressure also
decrease (e.g. if first evap is at 1 atm the last evap. will be under vacuum).
Dr SMS 2012/2013
23
steam, T
S
feed, T
F
concentrate
from first
effect.
vapor T
1
(1)
T
1
(2)
T
2
(3)
T
3
concentrate
from second
effect.
concentrated
product
condensate
vapor T
2
vapor T
3
to vacuum
condenser
1 kg of steam will evaporate 1 kg of
water in each evaporation
The 1
st
evap. operates at a T high
enough that the evaporated water
serves as the heating medium to the
2
nd
evap.
Very rough estimation, 3kg water will
be evaporated for 1 kg steam
Steam economy (kg vapor
evaporated/kh steam used) is
increased
+
Backward Feed Multiple Effect Evaporators
Fresh feed enters the last and coldest effect and continues on until the
concentrated product leaves the first effect.
Advantageous when the fresh feed is cold or when concentrated product
is highly viscous.
Liquid pump are used in each effects, since the flow is from low to high
pressure.
The high temperature in the first effect reduce the viscosity and give
reasonable heat-transfer coefficient.
Dr SMS 2012/2013
24
steam, T
S
feed, T
F
vapor T
1
(1)
T
1
(2)
T
2
(3)
T
3
concentrated
product
condensate
vapor T
2
vapor T
3
to vacuum
condenser
+
Parallel Feed Multiple Effect Evaporators
Involves the adding of fresh feed to each effect and the
withdraw of concentrated product from each effect.
However, the vapor from each effect is still used to heat the
next effect
Mainly used when the feed is almost saturated and solid crystal
are the product, as in the evaporation of brine to make salt
Dr SMS 2012/2013
25
+
Overall Heat Transfer Coefficients in Evaporator
Components contribute to the overall heat transfer coefficient , U in evaporator
steam-side condensing coefficient can be predicted using Eqs 4.8-20 to 4.8-26.
metal wall resistance usually negligible due to high thermal conductivity of metal;
increase velocity to decrease the rate of scale formation
resistance of the scale on the liquid side cannot be predicted
liquid film coefficient, h - usually inside the tube - can be predicted using various eq
depend on type of tubes configuration/evaporator type
Dr SMS 2012/2013
26
+
Calculation Method for Single Effect Evaporator
h
F
and h
L
often not available, enthalpy-concentration data are available for only few substance,
some approximation are made:
Using latent heat of evaporation of 1 kg water from from steam table at solution boiling temperature, T
1
Calculate using heat capacity, c
pF
and c
pL
if available
Dr SMS 2012/2013
27
MATERIAL BALANCE
Total mass balance
F = L+V
Balance on solute/solids
Fx
F
= Lx
L
ENERGY BALANCE
Heat in feed + Heat in steam = Heat in concentrated liquid + Heat in vapor + Heat in condensed steam
Fh
F
+SH
s
= Lh
L
+VH
v
+Sh
s
Fh
F
+S = Lh
L
+VH
v
; is latent heat of steam ( =H
s
-h
s
)
Heat transfer to the evaporator
q = S(H
s
-h
s
)=S
Also general design eqution for evaporator
q = UA T =UA(T
S
T
1
)
+
Example 8.4-1
Heat-Transfer Area in Single-Effect Evaporator.
A continuous single-effect evaporator concentrates 9072 kg/h of a 1.0 wt % salt
solution entering at 311.0 K (37.8 C) to a final concentration of 1.5 wt %. The
vapor space of the evaporator is at 101.325 kPa (1.0 atm abs) and the steam
supplied is saturated at 143.3 kPa. The overall coefficient U = 1704 W/m2 .K.
calculate the amounts of vapor and liquid product and the heat-transfer area
required. Assumed that, since it its dilute, the solution has the same boiling point
as water.
Dr SMS 2012/2013
28
U = 1704 W/m
2
T
1
A = ?
P
1
= 101.325 kPa
F = 9072 kg/h
T
F
= 311 K
x
F
= 0.01
h
F
.
S , T
S
, H
S
P
S
= 143.3 kPa
L = ?
T
1
, h
L
x
L
= 0.015
S, T
S
, h
S
V = ?
T
1
, y
V
, H
V
+ Effect of Processing Variables on Evaporator
Operation.
Feed temperature, T
F
T
F
< T
bp
, some of latent heat of steam will be used to heat up the
cold feed, only the rest of the latent heat of steam will be used to
vaporize the feed.
feed is under pressure & T
F
> T
bp
, additional vaporization obtained
by flashing of feed.
Evaporator pressure, P
1
desirable T [q = UA(T
S
T
1
)], A & cost .
T
1
depends on P
1 -
will P
1
T
1
then T (e.g under vacuum) .
Steam pressure, P
S
P
S
will T
S
but high-pressure steam is costly.
Optimum T
S
by overall economic balances are need.
Dr SMS 2012/2013
29
+
Boiling Point Rise & Heat of Solution
Majority cases, solutions in evaporator are not dilute, thus
thermal properties of the solution being evaporated may differ
considerably with water.
Dhrings rule a straight line of solution boiling point against
water boiling point at the same pressure for a given
concentration at different pressures
Heat of solution must be considered in heat balance for the
substance that give a considerable temperature rise during
dissolve in water.
Dr SMS 2012/2013
30
+
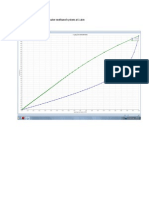
Duhrings Plot
Dr SMS 2012/2013
31
+
Example 8.4-2
Dr SMS 2012/2013
32
+
Enthalpy-Concentration Chart
Dr SMS 2012/2013
33
+
Example 8.4-3
An evaporator is used to concentrate 4536 kg/h of a 20 % solution of
NaOH in water entering at 60 C to a product of 50 % solid. The
pressure of the saturated steam used is 172.4 kPa and the pressure
in the vapor space of the evaporator is 11.7 kPa. The overall heat-
transfer coefficient is 1560 W/m2.K. calculate the steam used, the
steam economy in kg vaporized/kg steam used, and the heating
surface area in m2
Dr SMS 2012/2013
34
U = 1560 W/m
2
T
1
A = ?
P
1
= 11.7 kPa
F = 4536 kg/h
T
F
= 60 C
x
F
= 0.2
h
F
.
S = ?
T
S
, H
S
P
S
= 172.4 kPa
L, T
1
, h
L
x
L
= 0.5
S, T
S
, h
S
V, T
1
, H
V
+
Solution Example 8.4-3
Refer to Fig. 8.4-4, for flow diagram for this solution.
For the total balance, F = 4536 = L + V
For the balance on the solute alone, F x
F
= L x
L
4536 (0.2) = L (0.5)
L =1814 kg/h of liquid
Substituting into total balance and solving,
V =2722 kg/h of vapor
To determine T
1
= T
sat
+ BPR
of the 50 % concentrate product, first we obtain T
sat
of pure water from steam table. At 11.7 kPa, T
sat
= 48.9 C.
From Duhring chart (Fig. 8.4-2), for a T
sat
= 48.9 C and 50 % NaOH , the boiling
point of the solution is T
1
= 89.5 C.
From the enthalpy-concentration chart (Fig.8.4-3), for
T
F
= 60 C and x
F
= 0.2 get h
F
= 214 kJ/kg.
T
1
= 89.5 C and x
L
= 0.5 get h
L
= 505 kJ/kg.
Dr SMS 2012/2013
35
+
Solution Example 8.4-3
For saturated steam at 172.4 kPa, from steam table, we get
T
S
= 115.6 C and = 2214 kJ/kg.
To get H
V
for superheated vapor, first we obtain the enthalpy at T
sat
= 48.9 C and P
1
= 11.7
kPa, get H
sat
= 2590 kJ/kg.
Then using heat capacity of 1.884 kJ/kg.K for superheated steam. So
H
V
= H
sat
+ c
P
BPR = 2590 + 1.884 (40.6)
= 2667 kJ/kg. (alternatively read superheated table)
Substituting into heat balance equation and solving for S,
F h
F
+ S = L h
L
+ V H
V
4535 (214) + S (2214) = 1814 (505) + 2722 (2667)
S =3255 kg steam /h.
The heat q transferred through the heating surface area, A is
q = S () = 3255 (2214) (1000 / 3600) = 2 002 000 W
Solving for capacity single-effect evaporator equation;
q = U A T = U A (T
S
T
1
)
2 002 000 = 1560 A (115.6 89.5)
Solving, A =49.2 m
2
. Steam economy =2722/3255 = 0.836
Dr SMS 2012/2013
36
+
Calculation Method for Multiple Effect Evaporator
The calculation are done using material
balance, heat balance and heat capacity
equation (q=UAT) for each effect.
Normally using trial and error method.
Objective to calculate
Area (A) in each effect
Amount of steam (S) need
Amount of vapor (V) leaving each
effect
Usually given or known value
Steam pressure in first effect
Final pressure in the vapor space of last
effect (P3)
First condition and flow to first effect (F, X
F
)
Final concentration of the liquid leaving on
the last effect (X
3
)
Physical properties such as enthalpies or
heat capacity of the liquid and vapor
Overall heat transfer coefficient on each
effect, normally the value is same in each
effect, U
37
Dr SMS 2012/2013
(3)
U3
(2)
U2
S
P
S1
T
3
T
1
T
2
F
x
F
T
F
T
1
, L
1
, x
1
V
1
= F L
1
(1)
U1
V
2
= L
1
L
2
V
3
= L
2
L
3
T
S1
T
S3
T
S2
T
2
, L
2
, x
2
T
3
L
3
x
3
P
3
+ Calculation Method for Multiple Effect
Evaporator
Assumption made in operation;
no boiling point rise.
no heat of solution.
neglecting the sensible heat necessary to heat the feed to the boiling point.
Heat balances for multiple/triple-effect evaporator.
Heat is same in all effect: q =U
1
A
1
T
1
=U
2
A
2
T
2
=U
3
A
3
T
3
Areas in all effects are equal,: q/A = U
1
T
1
= U
2
T
2
= U
3
T
3
The temperature drops in evaporator (no BPR),
T = T
1
+ T
2
+ T
3
= T
S
T
3
The temperature drops in evaporator (with BPR),
T = T
1
+ T
2
+ T
3
= T
S
T
sat@P3
(BPR
1
+BPR
2
+BPR
3
)
hence we know that T are approximately inversely proportional to the values of U,
similar equations can be written for T
2
and T
3
if we assumed that the value of U is the same in each effect, the capacity equation,
q = U A (T
1
+ T
2
+ T
3
) = UA T
Dr SMS 2012/2013
38
3 2 1
1
1
1 1 1
1
U U U
U
T T
+
Calculation Method for Multiple Effect Evaporator
For the given x
3
and P
3
and find
BPR
3
if exist
From an overall MB , determine V
T
= V
1
+ V
2
+ V
3
(1
st
trial assumption V
1
=V
2
=V
3
)
Calculate the amount of
concentrated solutions (L
1
,L
2
,L
3
) &
their concentrations (X
1
,X
2
,X
3
) in
each effect using MB
Find BPR & T in each
effect & T.
If the feed is very cold, the
portions may be modified
appropriately, calculate the
boiling point in each effect.
Calculate V and L in each effect
through MEB
If the amounts differ significantly
from the assumed values in step 2;
step 2,3 and 4 must be repeated with
the amounts just calculated.
Using heat transfer equations for
each effect, calculate A required
for each effect. Then calculate A
m
= (A
1
+A
2
+A
3
)/3. Repeat second
trial if the area is not reasonably
close to each other
For second trial, using new
value of L1,L2,L3, V1,V2,V3
and calculated solid
concentration in each effect
Obtain new values T
1
=
T
1
A
1
/A
m
, , T
2
, T
3
, then
determine new T for find new are
as step 4.
Dr SMS 2012/2013
39
+
Dr SMS 2012/2013
40
Find T
3
, BPR
3
and T
S3
Assume V
1
=V
2
=V
3
Calc. L
1
,L
2
,L
3
,X
1
,X
2
,X
3
from MB
Compare A
1
,A
2
,A
3
with A
m
Calc. q
1
, q
2
, q
3
and solve A
1
,A
2
,A
3
Find A
m
Compare V
1
,V
2
,V
3
from MB with V
1
,V
2
,V
3
from EB
Find H
1
,H
2
,H
3
,
s1
,
s2
,
s3
Find T
1
,T
2
,T
3
,T
s1
,T
s2
,T
s3
Calc. T, T
1
, T
2
, T
3
Adjust for cold feed
Calc. BPR
1
, BPR
2
, BPR
3
From EB, calc. new V
1
,V
2
,V
3
, L
1
,L
2
,L
3
,
STOP
>10%
>10%
+
Example 8.5-1
A triple-effect forward-feed evaporator is being used to evaporate a sugar solution
containing 10 wt% solids to a concentrated solution of 50 %. The boiling-point
rise of the solutions (independent of pressure) can be estimated from (BPR C
= 1.78x + 6.22 x
2
), where x is wt fraction of sugar in solution. Saturated steam
at 205.5 kPa and 121.1C saturation temperature is being used. The pressure
in the vapor space of the third effect is 13.4 kPa. The feed rate is 22 680 kg/h
at 26.7 C. the heat capacity of the liquid solutions is c
P
= 4.19 2.35x kJ/kg.K.
The heat of solution is considered to be negligible. The coefficients of heat
transfer have been estimated as U
1
= 3123, U
2
= 1987, and U
3
= 1136 W/m
2
.K.
If each effect has the same surface area, calculate the area, the steam rate
used, and the steam economy.
Dr SMS 2012/2013
41
(3)
U3=1136
(2)
U2=1987
S = ?
T
S1
= 121.1 C
P
S1
= 205.5 kPa
T
3
T
1
T
2
F = 22680
x
F
= 0.1
T
F
= 26.7 C
T
1
, L
1
, x
1
V
1
= 22,680 L
1
(1)
U1=3123
V
2
= L
1
L
2
V
3
= L
2
- 4536
T
S1
T
S3
T
S2
T
2
, L
2
, x
2
T
3
L
3
= 4536
x
3
= 0.5
P
3
= 13.4 kPa
+
Dr SMS 2012/2013
42
(3)
U3=1136
(2)
U2=1987
S = ?
T
S1
= 121.1 C
P
S1
= 205.5 kPa
T
3
T
1
T
2
F = 22680
x
F
= 0.1
T
F
= 26.7 C
T
1
, L
1
, x
1
V
1
= 22,680 L
1
(1)
U1=3123
V
2
= L
1
L
2
V
3
= L
2
- 4536
T
S1
T
S3
T
S2
T
2
, L
2
, x
2
T
3
L
3
= 4536
x
3
= 0.5
P
3
= 13.4 kPa
A triple-effect forward-feed evaporator is being
used to evaporate a sugar solution containing
10 wt% solids to a concentrated solution of 50
%. The boiling-point rise of the solutions
(independent of pressure) can be estimated
from (BPR C = 1.78x + 6.22 x
2
), where x is wt
fraction of sugar in solution. Saturated steam at
205.5 kPa and 121.1C saturation temperature
is being used. The pressure in the vapor space
of the third effect is 13.4 kPa. The feed rate is
22 680 kg/h at 26.7 C. the heat capacity of the
liquid solutions is c
P
= 4.19 2.35x kJ/kg.K. The
heat of solution is considered to be negligible.
The coefficients of heat transfer have been
estimated as U
1
= 3123, U
2
= 1987, and U
3
=
1136 W/m
2
.K. If each effect has the same
surface area, calculate the area, the steam rate
used, and the steam economy.
Fi nd T
3
, BPR
3
and T
S3
Assume V
1
=V
2
=V
3
Cal c. L
1
,L
2
,L
3
,X
1
,X
2
,X
3
from MB
Compare A
1
,A
2
,A
3
wi th A
m
Cal c. q
1
, q
2
, q
3
and sol ve A
1
,A
2
,A
3
Fi nd A
m
Compare V
1
,V
2
,V
3
from MB wi th V
1
,V
2
,V
3
from EB
Fi nd H
1
,H
2
,H
3
,
s1
,
s2
,
s3
Fi nd T
1
,T
2
,T
3
,T
s1
,T
s2
,T
s3
Cal c. T, T
1
, T
2
, T
3
Adj ust for col d feed
Cal c. BPR
1
, BPR
2
, BPR
3
From EB, cal c. new V
1
,V
2
,V
3
, L
1
,L
2
,L
3
,
STOP
>10%
>10%
+
Summary
Introduction
Processing Factor in Evaporation
Type of Evaporation
Method of Operation of Evaporators
Calculation Method for Single Effect Evaporator
Calculation Method for Multiple Effect Evaporator
Dr SMS 2012/2013
43
Any Question?
S-ar putea să vă placă și
- Chapter 2 Evaporation PDFDocument54 paginiChapter 2 Evaporation PDFMaribel Moreno0% (2)
- Evaporator HistoryDocument7 paginiEvaporator History박우진Încă nu există evaluări
- EvaporationDocument37 paginiEvaporationVimal KumarÎncă nu există evaluări
- Evaporator HistoryDocument7 paginiEvaporator History박우진Încă nu există evaluări
- Energetics: Coffee Pharmaceutical Enzymes Kraft PulpingDocument6 paginiEnergetics: Coffee Pharmaceutical Enzymes Kraft Pulping박우진Încă nu există evaluări
- Energetics: Pharmaceutical Enzymes Kraft PulpingDocument6 paginiEnergetics: Pharmaceutical Enzymes Kraft Pulping박우진Încă nu există evaluări
- Energetics: Solvents Desalination Zero Liquid Discharge Drinking WaterDocument6 paginiEnergetics: Solvents Desalination Zero Liquid Discharge Drinking Water박우진Încă nu există evaluări
- KKKR3723 20182019 Utility Part 4 - EvaporationDocument56 paginiKKKR3723 20182019 Utility Part 4 - EvaporationPutriÎncă nu există evaluări
- Double Effect EvaporatorDocument10 paginiDouble Effect Evaporatorgeek3112100% (7)
- Evaporator: How An Evaporator WorksDocument3 paginiEvaporator: How An Evaporator WorksJames VincentÎncă nu există evaluări
- 4KKKR3723 Part Iv-20200217084742 PDFDocument56 pagini4KKKR3723 Part Iv-20200217084742 PDFPrince Yogadevan VijayanÎncă nu există evaluări
- UntitledDocument17 paginiUntitledSanjay v.r v.rÎncă nu există evaluări
- Evaporators PDFDocument25 paginiEvaporators PDFMunibah AhsanÎncă nu există evaluări
- Chapter OneDocument23 paginiChapter OneAyuob ElsharefÎncă nu există evaluări
- 2.1 Objective of EvaporationDocument8 pagini2.1 Objective of EvaporationwakÎncă nu există evaluări
- EVAPORATORDocument8 paginiEVAPORATORyilma wendayehuÎncă nu există evaluări
- EVAPORATOR FinalDocument86 paginiEVAPORATOR FinalArgha TalukderÎncă nu există evaluări
- Chapter OneDocument27 paginiChapter OneAyuob ElsharefÎncă nu există evaluări
- Prepared By: Hussein Maytham Saied: EvaporationDocument12 paginiPrepared By: Hussein Maytham Saied: Evaporationحسين ميثم سعيد مهديÎncă nu există evaluări
- FPE20306-2019 - Chapter 3 - Evaporation - TheoryDocument18 paginiFPE20306-2019 - Chapter 3 - Evaporation - TheoryAntonio MoncayoÎncă nu există evaluări
- Chapter 21: Evaporation - Principles, Types of EvaporatorsDocument7 paginiChapter 21: Evaporation - Principles, Types of EvaporatorssdfaÎncă nu există evaluări
- Evaporation and DehydrationDocument23 paginiEvaporation and DehydrationRogelyn JosolÎncă nu există evaluări
- Rising Film EvaporatorsDocument10 paginiRising Film EvaporatorsPelin Yazgan BirgiÎncă nu există evaluări
- Evapn NotesDocument12 paginiEvapn NotesShubham bangarÎncă nu există evaluări
- EvaporatorDocument7 paginiEvaporatormohitÎncă nu există evaluări
- Sch1210 Heat Transfer Unit V Ii/Iv EvaporationDocument19 paginiSch1210 Heat Transfer Unit V Ii/Iv EvaporationLearning AspirationÎncă nu există evaluări
- Evaporator SDocument23 paginiEvaporator SMehwish NoorÎncă nu există evaluări
- PTT205 Heat and Mass Transfer: EvaporatorDocument43 paginiPTT205 Heat and Mass Transfer: Evaporatorkkk100% (1)
- Operation Evaporation ToolsDocument41 paginiOperation Evaporation Toolsati atun chasanahÎncă nu există evaluări
- Evaporators: in Evaporation Physical and Chemical Properties of The Solution Being ConcentratedDocument19 paginiEvaporators: in Evaporation Physical and Chemical Properties of The Solution Being ConcentrateddigecaÎncă nu există evaluări
- Heat and Mass TransferDocument23 paginiHeat and Mass TransferMaria Cecille Sarmiento GarciaÎncă nu există evaluări
- Forced Circulation EvaporatorDocument8 paginiForced Circulation EvaporatorNIDHARSHANA S100% (2)
- 3.1 Evaporation1Document21 pagini3.1 Evaporation1Mukhzin RashidÎncă nu există evaluări
- Unit V EvaporationDocument23 paginiUnit V EvaporationpavijayaÎncă nu există evaluări
- Fe 248 Uofp II NotesDocument26 paginiFe 248 Uofp II NotesSumit NirmalÎncă nu există evaluări
- Evaporation-An IntroductionDocument23 paginiEvaporation-An IntroductionKusmakarÎncă nu există evaluări
- 4 - Evaporation SDocument32 pagini4 - Evaporation Sabdelsalam kasemÎncă nu există evaluări
- Upload: Login SignupDocument16 paginiUpload: Login SignupSeid AragawÎncă nu există evaluări
- EvaporationDocument59 paginiEvaporationMonty KushwahaÎncă nu există evaluări
- RefluxDocument5 paginiRefluxFranco Salerno100% (1)
- Heat Transfer Equipment EvaporatorDocument57 paginiHeat Transfer Equipment EvaporatorAsadulhaq Ali HamidiÎncă nu există evaluări
- Climbing Film GanganDocument20 paginiClimbing Film GanganAdeniran Joshua50% (2)
- DryingDocument8 paginiDryingAjaya Kumar MohapatraÎncă nu există evaluări
- Dr. Shina Gautam Associate Professor, Chemical Engineering, Shroff S. R. Rotary Institute of Chemical TechnologyDocument45 paginiDr. Shina Gautam Associate Professor, Chemical Engineering, Shroff S. R. Rotary Institute of Chemical TechnologyJITENDRA CARPENTERÎncă nu există evaluări
- Types of SteamDocument6 paginiTypes of SteamMohammad Ali ZamanÎncă nu există evaluări
- Evap DesignDocument16 paginiEvap DesignAhmed Ali100% (3)
- 04 EvaporationDocument36 pagini04 EvaporationPharmacistBDÎncă nu există evaluări
- Evaporation FullDocument57 paginiEvaporation FullMonty KushwahaÎncă nu există evaluări
- Freeze Drying GuideDocument12 paginiFreeze Drying GuidePedro CostaÎncă nu există evaluări
- EVAPORATIONDocument50 paginiEVAPORATIONSanya SharmaÎncă nu există evaluări
- Boiling Point and DistillationDocument21 paginiBoiling Point and Distillationشهد إيادÎncă nu există evaluări
- DehydrationGROUP 5Document58 paginiDehydrationGROUP 5melannie adanteÎncă nu există evaluări
- Evaporator and Its TypesDocument22 paginiEvaporator and Its TypesIrfan ShafiqÎncă nu există evaluări
- Evaporation 2021 22Document69 paginiEvaporation 2021 22xnzrjp55tsÎncă nu există evaluări
- Evaporation: Central Institute of Technology KokrajharDocument42 paginiEvaporation: Central Institute of Technology KokrajharkennethmsorianoÎncă nu există evaluări
- Types of EvaporatorsDocument12 paginiTypes of Evaporatorsapi-377437388% (8)
- Types of Evaporators: LTV Rising-Film LTV Falling-Film Forced-Circulation EvaporatorsDocument12 paginiTypes of Evaporators: LTV Rising-Film LTV Falling-Film Forced-Circulation EvaporatorsAldair Vergel RangelÎncă nu există evaluări
- PERFORMANCE TEST ON VAPOUR COMPRESSION REFRIGERATION SYSTEM USING R290 & R134a MIXTUREDocument7 paginiPERFORMANCE TEST ON VAPOUR COMPRESSION REFRIGERATION SYSTEM USING R290 & R134a MIXTUREJASH MATHEWÎncă nu există evaluări
- Evaporator PresntationDocument22 paginiEvaporator Presntationzubi13100% (2)
- Process Engineering: Facts, Fiction and FablesDe la EverandProcess Engineering: Facts, Fiction and FablesEvaluare: 3 din 5 stele3/5 (2)
- BKG 3433 Transmission & DistributionDocument3 paginiBKG 3433 Transmission & DistributionAzmiHafifiÎncă nu există evaluări
- W2 PID STDDocument12 paginiW2 PID STDAzmiHafifiÎncă nu există evaluări
- Exercise 4 PDC Sem II 201415 STDDocument1 paginăExercise 4 PDC Sem II 201415 STDAzmiHafifiÎncă nu există evaluări
- ABSTARCT Exp5Document1 paginăABSTARCT Exp5AzmiHafifiÎncă nu există evaluări
- Quarterly Result 20090731Document12 paginiQuarterly Result 20090731AzmiHafifiÎncă nu există evaluări
- A) T-Xy Diagram For The Water-Methanol System at 1 AtmDocument2 paginiA) T-Xy Diagram For The Water-Methanol System at 1 AtmAzmiHafifiÎncă nu există evaluări
- Schedule of PD 1 Workshop - Aspen & SuperProDocument1 paginăSchedule of PD 1 Workshop - Aspen & SuperProAzmiHafifiÎncă nu există evaluări
- IntroductionDocument4 paginiIntroductionAzmiHafifiÎncă nu există evaluări
- Front PageDocument3 paginiFront PageAzmiHafifiÎncă nu există evaluări
- Faculty of Chemical and Natural Resources Engineering: Research Proposal (20%)Document2 paginiFaculty of Chemical and Natural Resources Engineering: Research Proposal (20%)AzmiHafifiÎncă nu există evaluări
- (Selasa) : BIL Nama No. Matrik Alamat KolejDocument2 pagini(Selasa) : BIL Nama No. Matrik Alamat KolejAzmiHafifiÎncă nu există evaluări
- Minor Report From Proposal PresentationDocument2 paginiMinor Report From Proposal PresentationAzmiHafifiÎncă nu există evaluări
- (A) Generate A Txy Diagram For The Water-Methanol System at 1 AtmDocument4 pagini(A) Generate A Txy Diagram For The Water-Methanol System at 1 AtmAzmiHafifiÎncă nu există evaluări
- Link GoldDocument1 paginăLink GoldAzmiHafifiÎncă nu există evaluări
- Link GoldDocument1 paginăLink GoldAzmiHafifiÎncă nu există evaluări
- Roles of Engineer in Herbal Industy AND Warisan Kundur Resources SDN BHDDocument2 paginiRoles of Engineer in Herbal Industy AND Warisan Kundur Resources SDN BHDAzmiHafifiÎncă nu există evaluări
- Buku Log Briged Siswa (UQB1011) Semester 1 Sesi 2011/2012Document1 paginăBuku Log Briged Siswa (UQB1011) Semester 1 Sesi 2011/2012AzmiHafifiÎncă nu există evaluări
- ConclusionsDocument2 paginiConclusionsAzmiHafifiÎncă nu există evaluări
- Moringa Oil CharacteristicsDocument13 paginiMoringa Oil CharacteristicsAzmiHafifiÎncă nu există evaluări
- #Design Equation D (X) /D (W) (-Raprime) / Fao X (0) 0 X (F) 0.70 W (0) 0Document2 pagini#Design Equation D (X) /D (W) (-Raprime) / Fao X (0) 0 X (F) 0.70 W (0) 0AzmiHafifiÎncă nu există evaluări
- Section 4: Chemical Reaction Engineering IiDocument1 paginăSection 4: Chemical Reaction Engineering IiAzmiHafifiÎncă nu există evaluări
- IntroductionDocument7 paginiIntroductionAzmiHafifiÎncă nu există evaluări
- #Design Equation D (X) /D (W) (-Raprime) / Fao X (0) 0 X (F) 0.70 W (0) 0Document2 pagini#Design Equation D (X) /D (W) (-Raprime) / Fao X (0) 0 X (F) 0.70 W (0) 0AzmiHafifiÎncă nu există evaluări
- Exp 1 DLTDocument5 paginiExp 1 DLTSurenthran SundarÎncă nu există evaluări
- Chapter 1 - Modelling, Computers and Error AnalysisDocument35 paginiChapter 1 - Modelling, Computers and Error AnalysisAzmiHafifiÎncă nu există evaluări
- Final Report Format 2014AAAAAADocument18 paginiFinal Report Format 2014AAAAAAAzmiHafifiÎncă nu există evaluări
- File LapDocument1 paginăFile LapAzmiHafifiÎncă nu există evaluări
- Final Report Format 2014AAAAAADocument18 paginiFinal Report Format 2014AAAAAAAzmiHafifiÎncă nu există evaluări
- Exp 1 DLTDocument5 paginiExp 1 DLTSurenthran SundarÎncă nu există evaluări
- Lectures 8 9 10Document185 paginiLectures 8 9 10AzmiHafifiÎncă nu există evaluări
- EVAPORATORDocument8 paginiEVAPORATORyilma wendayehuÎncă nu există evaluări
- Unit-Ii 13Document10 paginiUnit-Ii 13Sabari RajanÎncă nu există evaluări
- AP Government GATE Online Classes: Day-10 (04.06.2020) Dr. R. Srikanth Professor ANITS-VisakhapatnamDocument74 paginiAP Government GATE Online Classes: Day-10 (04.06.2020) Dr. R. Srikanth Professor ANITS-VisakhapatnamRajmangal NishadÎncă nu există evaluări
- Types of EvaporatorsDocument12 paginiTypes of Evaporatorsapi-377437388% (8)
- Types of Evaporators: LTV Rising-Film LTV Falling-Film Forced-Circulation EvaporatorsDocument12 paginiTypes of Evaporators: LTV Rising-Film LTV Falling-Film Forced-Circulation EvaporatorsAldair Vergel RangelÎncă nu există evaluări
- Climbing FilmDocument34 paginiClimbing FilmTunji Aminu100% (1)
- Paper No.: 04 Paper Title: Unit Operations in Food Processing Module-14:EvaporationDocument9 paginiPaper No.: 04 Paper Title: Unit Operations in Food Processing Module-14:EvaporationKanchanÎncă nu există evaluări
- Lopez-Toledo - Heat and Mass Transfer Characteristics of A WipedDocument224 paginiLopez-Toledo - Heat and Mass Transfer Characteristics of A WipedNatrix2Încă nu există evaluări
- Vinasse Concentration and IncinerationDocument25 paginiVinasse Concentration and Incinerationtsrinivasan508350% (2)
- GEA Evaporation-Technology Brochure EN tcm11-16319 PDFDocument24 paginiGEA Evaporation-Technology Brochure EN tcm11-16319 PDFItsartÎncă nu există evaluări
- EvaporatorDocument7 paginiEvaporatormohitÎncă nu există evaluări
- EvaporationDocument7 paginiEvaporationPoonatiGiriÎncă nu există evaluări
- EvaporatorDocument19 paginiEvaporatortalhawasim50% (2)
- Chapter9 Evaporation SystemDocument86 paginiChapter9 Evaporation Systemngoc.nguyenlamÎncă nu există evaluări
- Unit Operations in Chemical IndustriesDocument20 paginiUnit Operations in Chemical IndustriesVicky AgroÎncă nu există evaluări
- Dubai-IIE Nr. 4 Self Cleaning Heat ExchangerDocument15 paginiDubai-IIE Nr. 4 Self Cleaning Heat ExchangerevrimkÎncă nu există evaluări
- MCQ QuestionsDocument29 paginiMCQ Questionsj setty70% (63)
- Heat and Mass TransferDocument23 paginiHeat and Mass TransferMaria Cecille Sarmiento GarciaÎncă nu există evaluări
- Evaporation JK From GavhaneDocument21 paginiEvaporation JK From GavhaneRADHE GRAPHICSÎncă nu există evaluări
- Evaporators FinalDocument32 paginiEvaporators FinalAhmad ButtÎncă nu există evaluări
- Unit V EvaporationDocument23 paginiUnit V EvaporationpavijayaÎncă nu există evaluări
- MCQ Sohel SirDocument10 paginiMCQ Sohel SirPulok HasanÎncă nu există evaluări
- Evaporator SDocument23 paginiEvaporator SMehwish NoorÎncă nu există evaluări
- 4 - Evaporation SDocument32 pagini4 - Evaporation Sabdelsalam kasemÎncă nu există evaluări
- Chapter04Shell and Tube Heat Exchanger - All - EmbeddedDocument71 paginiChapter04Shell and Tube Heat Exchanger - All - EmbeddedSiddharth MohapatraÎncă nu există evaluări
- Forced Circulation Evaporator Final ReportDocument40 paginiForced Circulation Evaporator Final ReportManvi SharmaÎncă nu există evaluări
- Evaporator Design CalculationDocument58 paginiEvaporator Design CalculationManoj BÎncă nu există evaluări
- High PurityMediaDocument8 paginiHigh PurityMediaformaksicÎncă nu există evaluări
- Sugar Cane Liquid ConcentratedDocument9 paginiSugar Cane Liquid ConcentratedFrank DagohoyÎncă nu există evaluări
- Foul Condensate8Document8 paginiFoul Condensate8Arun YogaÎncă nu există evaluări