Documente Academic
Documente Profesional
Documente Cultură
2015 SPM Exams Tips and Prediction - Chemistry
Încărcat de
Ganesh0 evaluări0% au considerat acest document util (0 voturi)
32 vizualizări14 paginispm
Titlu original
2015 Spm Exams Tips and Prediction - Chemistry
Drepturi de autor
© © All Rights Reserved
Formate disponibile
PPTX, PDF, TXT sau citiți online pe Scribd
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentspm
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PPTX, PDF, TXT sau citiți online pe Scribd
0 evaluări0% au considerat acest document util (0 voturi)
32 vizualizări14 pagini2015 SPM Exams Tips and Prediction - Chemistry
Încărcat de
Ganeshspm
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PPTX, PDF, TXT sau citiți online pe Scribd
Sunteți pe pagina 1din 14
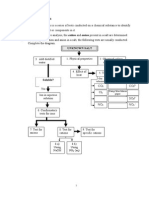
2015 SPM EXAMS TIPS
AND PREDICTION CHEMISTRY
Form 4 Chapter 2 & 3
Able
to explain the diffusion of bromine particles
throughout the two gas jar.
Master
all the calculation from chapter 3 and
chapter 7 If come out in essay, marks given is
between 4 m 6 m for calculation involving
interpretation of chemical equation!!
Describe
Experiment to determine empirical
formula of magnesium oxide and copper oxide
Not in Essay section before!
Experiment
to determine the melting point of
substance X - Not in essay section for long time!
Form 4 Chapter 4 Spot in Structure and Paper 3!!
[2014
chapter 4: Essay section C : Determine basic oxide,
amphoteric oxide and acidic oxide - just small part only]
(*Long
time not set in essay part since 2006)
Practice all the balanced chemical equations for all the
reactions in group 1 and group 17
Must able to explain the difference reactivity of elements
when going down the group 1 and group 17
Remember to put the word of *ATOM* OR *MOLECULE* for
the explanation
Eg : Sodium atom has a higher tendency to donate its
Form 4 chapter 5 (Chemical Bonds) Spot in Essay!!
[2014 chapter 5 : Structure Section A - Physical properties of
ionic and covalent compound (small part only]
Explain the formation of ionic bond and covalent bond +
draw the electron arrangement
Students to read the marking scheme
Able to draw electron arrangement of ionic compound and
covalent compound correctly
Explain the differences of physical property of ionic
compound and covalent compound from aspect electrical
conductivity and melting point
Experiment to study the electrical conductivity of ionic
compound and covalent compound in solid and molten state
Form 4 chapter 6 (Electrolysis) Spot in
structure/essay and PAPER 3!!
[2014 : Structure Section A - Electrolysis molten lead(II) bromide
and copper(II) sulphate solution]
Chemical cell (Structure)
Able to explain how to arrange metals based on potential
difference (essay part)
Describe electroplating/purification experiment (Have asked in
essay on 2010)
Able to compare and contrast chemical cell and electrolytic cell
(essay part)
Planning experiment paper 3 : Effect of type of electrode /
Electroplating
(recommend student to view Trial Terengganu paper 3 2014 and
Kelantan paper 3 2015)
Form 4 Chapter 7 (Acid and Bases) and 8 (Salt) Spot
in structure / essay!!
[2014
: Essay section B - explain different pH value of
strong acid and weak acid]
1.
Titration experiment and involve calculation
2.
Able to describe how to prepare standard solution
(essay part)
3.
Able to explain the differences of pH values between
strong acid and weak acid // strong alkali and weak
alkali
Form
4 Chapter 8 (SALTS) Spot in structure!!
[2014
: Essay Section B - Identify cation and anion]
Form 4 Chapter 9 (Manufactured Substances
In Industry) Spot in essay/P3!!
[2014
: Structure section A - Glass and polymer]
1.
Hardness of Alloy experiment essay part /
Paper 3 structure
2.
Preparation of ammonium nitrate salt essay
part
3.
Contact process or Haber process structure /
essay
4.
Alloy + Glass + Ceramic + Polymer + Composite
materials - structure / essay
Form 5 Chapter 10 (Rate of reaction) Spot in Essay !!
[2014 : Structure - Factor of temperature for the reaction between zinc
and hydrochloric acid]
1. COLLISION THEORY (very POPULAR) REMEMBER catalyst will not
increase frequency of collision but will increase frequency of effective
collision
***Factor of total surface area, concentration, temperature must
mention increase frequency of collision between [metal] atom and
hydrogen ion // [metal] carbonate(insoluble salt) and hydrogen ion //
carbonate ion(soluble salt) and hydrogen ion
2. Experiment effect of temperature / concentration on the r.o.r
between sodium thiosulphate solution and sulphuric acid (Paper 3
planning experiment)
3. Experiment of effect of catalyst on the rate of reaction (essay)
Form 5 Chapter 11 (Carbon compound) Spot
structure / essay!!
[2014 : Structure Section A and Paper 3 Planning
experiment]
1. CHEMICAL TEST TO DIFFERENTIATE ALKANE AND
ALKENE - Dont Use Combustion!!
2. Coagulation of latex AND Compare elasticity of
Vulcanised Rubber and Vulcanised Rubber (essay part)
3. Describe an experiment of dehydration reaction /
esterification reaction (essay part)
Essay Form 5 Chaper 12(Oxidation and Reduction)
Essay / Paper 3!!
[2014 : Sructure section A - Reduction of metal oxide by carbon
Essay section C - Describe an experiment of displacement of halogen]
1.U-tube EXPERIMENT (VERY POPULAR)
2.Experiment to compare reactivity of metal through reaction between
:-
(i) oxide of metal by hydrogen gas (Have asked in structure ONCE
on 2000)
(ii) metal with oxygen gas
4. Rusting of iron VERY POPULAR (may come out in paper 3 this
year!!)
5. Conversion Fe2+ to Fe3+ and vice versa (Essay part)
Form 5 Chapter 13 (Thermochemistry) Spot in
structure / Paper 3
[2014 : Essay Section C- Describe an experiment to
determine Heat of Neutralisation]
1. STUDY ALL!!! Please write the each Definition of Heat
precisely!!
2. Able to draw the energy level diagram correctly
3. Able to master all the calculation
4. HEAT OF combustion
Form 5 Chapter 14 (Chemicals for Consumer)
[2014 : None]
STUDY ALL!!
- Describe an experiment to study the effectiveness of
soap and detergent in hard water ( essay / paper 3
planning experiment)
Paper 3 Essay (17 marks)
Form 4
1. To study the acid and base properties of aluminium
oxide, sodium oxide and phosphorus pentoxide
2. Experiment to construct ES based on displacement of
metals
3. To study the factor of position ions/concentration
ions/type of electrode on the products of electrolysis
// Electroplating of metal
4. To compare the rate of rusting between iron, steel
and stainless steel
Paper 3 Essay (17 marks)
Form 5
1. Heat of Combustion
2. Experiment to study the factor of concentration /
temperature of sodium thiosulphate on r.o.r
3. Experiment to study the effect of amount of catalyst
on the rate of decomposition of hydrogen peroxide
4. Rusting of iron nail
5. Experiment to study the effectiveness of soap and
detergent in hard water
S-ar putea să vă placă și
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- Orange CakeDocument2 paginiOrange CakeGaneshÎncă nu există evaluări
- Chemistry STPM Sem 3 MSAB Pre-Trial QuestionDocument6 paginiChemistry STPM Sem 3 MSAB Pre-Trial QuestionKenneth Chan43% (7)
- Qualitative AnalysisDocument16 paginiQualitative AnalysisGaneshÎncă nu există evaluări
- Answer For Jadual BerkalaDocument8 paginiAnswer For Jadual BerkalaGaneshÎncă nu există evaluări
- Rancangan Tahunan Sains F1Document7 paginiRancangan Tahunan Sains F1GaneshÎncă nu există evaluări
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (588)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (400)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (74)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (121)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- Adequate Bearing Material and Heat TreatmentDocument20 paginiAdequate Bearing Material and Heat TreatmentdavideÎncă nu există evaluări
- 1504805126-HPI - CR-Series Copper Crusher - 04-2021ENDocument1 pagină1504805126-HPI - CR-Series Copper Crusher - 04-2021ENCaio BittencourtÎncă nu există evaluări
- Iso 9974-2Document4 paginiIso 9974-2willianÎncă nu există evaluări
- Misc Forrester SAP Competence CenterDocument16 paginiMisc Forrester SAP Competence CenterManuel ParradoÎncă nu există evaluări
- BMW X4 (2019-2022) Vs Audi Q5 Vs Land Rover Discovery Sport Vs Mercedes-Benz GLE - CarWaleDocument1 paginăBMW X4 (2019-2022) Vs Audi Q5 Vs Land Rover Discovery Sport Vs Mercedes-Benz GLE - CarWaleSahil GoyalÎncă nu există evaluări
- Eps Atc 0017 PDFDocument3 paginiEps Atc 0017 PDFSdreamworksÎncă nu există evaluări
- Lifting Plan For CranesDocument9 paginiLifting Plan For CranesBibin JohnÎncă nu există evaluări
- Research Papers in Mechanical Engineering Free Download PDFDocument4 paginiResearch Papers in Mechanical Engineering Free Download PDFtitamyg1p1j2Încă nu există evaluări
- ISO - 3601-2 O-Rings HousingDocument56 paginiISO - 3601-2 O-Rings HousingAlexey FlidliderÎncă nu există evaluări
- 9C606C.64 To 65Document24 pagini9C606C.64 To 65SHIRISHA YADAVÎncă nu există evaluări
- Planning For Information NetworkDocument32 paginiPlanning For Information NetworkChandraAdsenubiiÎncă nu există evaluări
- Heat Rate Calc in ExcelDocument13 paginiHeat Rate Calc in ExcelMukesh VadaliaÎncă nu există evaluări
- Strength of Materials Basics and Equations - Mechanics of Materials - Engineers EdgeDocument6 paginiStrength of Materials Basics and Equations - Mechanics of Materials - Engineers EdgeansarÎncă nu există evaluări
- Cloud Computing - Feb-Mar 2017Document40 paginiCloud Computing - Feb-Mar 2017Abhishek SinghÎncă nu există evaluări
- Technical Information: Chemical Resistance ChartDocument7 paginiTechnical Information: Chemical Resistance ChartthessandÎncă nu există evaluări
- NAWTEC18-3507: Comparison of Acid Gas Control Technologies in Efw FacilitiesDocument10 paginiNAWTEC18-3507: Comparison of Acid Gas Control Technologies in Efw FacilitiesPunki KokoÎncă nu există evaluări
- Seminar Report 2Document25 paginiSeminar Report 2DrMahendra Kumar Gokhroo0% (1)
- The Effects of Crankshaft Offset On The Engine FrictionDocument15 paginiThe Effects of Crankshaft Offset On The Engine Frictionqingcaohe100% (1)
- A New Finite Element Based On The Strain Approach For Linear and Dynamic AnalysisDocument6 paginiA New Finite Element Based On The Strain Approach For Linear and Dynamic AnalysisHako KhechaiÎncă nu există evaluări
- Pre-Spud Checklist # 4Document2 paginiPre-Spud Checklist # 4Yougchu LuanÎncă nu există evaluări
- Universal USB Installer - Easy As 1 2 3 - USB Pen Drive LinuxDocument4 paginiUniversal USB Installer - Easy As 1 2 3 - USB Pen Drive LinuxAishwarya GuptaÎncă nu există evaluări
- CST 336 Final Project Computown DocumentationDocument12 paginiCST 336 Final Project Computown Documentationapi-461214598Încă nu există evaluări
- QBDC - Season 5-RulebookDocument50 paginiQBDC - Season 5-RulebookHggvgÎncă nu există evaluări
- 2nd Term Physics ReviewerDocument5 pagini2nd Term Physics ReviewerAlfredo L. CariasoÎncă nu există evaluări
- CFJV00198BDocument360 paginiCFJV00198BCheongÎncă nu există evaluări
- PT 0817 Cebu Room Assignment PDFDocument16 paginiPT 0817 Cebu Room Assignment PDFPhilBoardResultsÎncă nu există evaluări
- Clevo M620ne-UDocument34 paginiClevo M620ne-UHh woo't hoofÎncă nu există evaluări
- TT2223 Week 12a Z-TransformDocument39 paginiTT2223 Week 12a Z-TransformAjiMaulanaÎncă nu există evaluări
- 165T-5 Parts ListDocument26 pagini165T-5 Parts ListJorge Luis Galezo MuñozÎncă nu există evaluări
- Elvax 460Document3 paginiElvax 460ingindjorimaÎncă nu există evaluări