Documente Academic
Documente Profesional
Documente Cultură
Lidoka in
Încărcat de
herzi rahmatul s0 evaluări0% au considerat acest document util (0 voturi)
57 vizualizări17 paginiwow
Titlu original
Lidoka In
Drepturi de autor
© © All Rights Reserved
Formate disponibile
PPTX, PDF, TXT sau citiți online pe Scribd
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentwow
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PPTX, PDF, TXT sau citiți online pe Scribd
0 evaluări0% au considerat acest document util (0 voturi)
57 vizualizări17 paginiLidoka in
Încărcat de
herzi rahmatul swow
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PPTX, PDF, TXT sau citiți online pe Scribd
Sunteți pe pagina 1din 17
Lidocaine
Lidocaine is a local anesthetic agent that also has
antiarrhythmic effects. It is a treatment for
ventricular tachycardia or ventricular fibrillation.
For patients who are more hemodynamically stable,
sustained monomorphic ventricular tachycardia due
to myocardial ischemia or infarction may be
successfully treated using lidocaine therapy.
Lidocaine therapy can also be considered for the
treatment of polymorphic ventricular tachycardia
due to myocardial ischemia or infarction.
Lidocaine inhibits transmembrane sodium influx into
the His-Purkinje fiber conduction system thereby
decreasing conduction velocity.
It also decreases the duration of the action potential
and as a result decreases the duration of the absolute
refractory period in Purkinje fibers and bundle of His.
Automaticity is decreased during lidocaine therapy.
The net effect of these cellular changes is that
lidocaine eradicates ventricular reentrant arrhythmias
by abolishing unidirectional blocks via increased
conduction through diseased fibers.
Therapeutic & Toxic
Concentrations
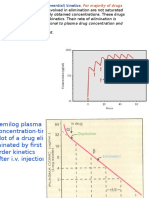
Lidocaine follows a two compartment model after
administered intravenously. This is especially
apparent when initial loading doses of lidocaine are
given as rapid intravenous injections over 15
minutes (max rate: 2550 mg/min) and a
distribution phase of 3040 minutes is observed
after drug administration.
Lidocaine moves rapidly from the blood into the
heart, and the onset of action is within a few
minutes after completion of the intravenous
injection. Because of these, the heart is considered
to be located in the central compartment of the
two-compartment model for lidocaine.
The generally accepted therapeutic range for
lidocaine is 1.55 g/mL.
In the upper end of the therapeutic range (>3
g/mL), some patients will experience minor side
effects including drowsiness, dizziness,
paresthesias, or euphoria.
Lidocaine serum concentrations above the
therapeutic range can cause muscle twitching,
confusion, agitation, dysarthria, psychosis,
seizures, or coma.
Cardiovascular adverse effects such as
atrioventricular block, hypotension, and circulatory
collapse have been reported at lidocaine
concentrations above 6 g/mL, but are not strongly
correlated with specific serum levels.
Clinical Pharmacokinetic Parameters
Lidocaine is almost completely eliminated by
hepatic metabolism (>95%). Hepatic metabolism is
mainly via the CYP3A enzyme system.
Monoethylglycinexylidide (MEGX) is the primary
metabolite resulting from lidocaine metabolism.
While a portion of MEGX is eliminated renally, most
of the metabolite is further converted hepatically to
glycinexylidide (GX) and other inactive metabolites.
GX is primarily eliminated by the kidney. MEGX and
GX have some antiarrhythmic activity (MEGX 80%
and GX 10%, relative to lidocaine), but have also
been implicated as the cause of some adverse
effects attributed to lidocaine therapy.
Lidocaine is usually given intravenously but may
also intramuscularly. After IM injection, absorption
is rapid and complete with maximum
concentrations occurring 1 hour after
administration and 100% bioavailability as long as
the patients peripheral circulation is not
compromised.
Oral absorption of lidocaine is nearly 100%.
However, lidocaine is extensively metabolized by
the CYP3A enzymes(intestinal wall and liver)
resulting in a large first-pass effect. Because
roughly 70% of an oral dose is converted to
metabolites, MEGX and GX concentrations are
high after oral administration of lidocaine
resulting in a high incidence of adverse effects.
Plasma protein binding in normal individuals is
70%. Of this value, approximately 30% is
bounded to albumin while 70% is bounded to 1-
acid glycoprotein (AGP).
AGP is secreted in large amounts in response to
certain stresses and disease states such as
trauma, heart failure, and myocardial infarction,
resulting in an unbound fraction as low as 10
15%.
The continuous increase in protein binding due to
AGP secretion causes a continuous decrease in
lidocaine clearance in patients with myocardial
infarction, and lidocaine concentrations can
accumulate to unexpectedly high levels.
Disease States and Conditions that
Alter Lidocaine Pharmacokinetics
Patients with liver cirrhosis or acute hepatitis have reduced
lidocaine clearance which results in a prolonged average
lidocaine half life of 5 hours.The mechanism for depressed
clearance in liver disease patients is destruction of liver
parenchyma where hepatic drug metabolizing enzymes are
present and reduction of liver blood flow.
The central volume of distribution and volume of
distribution for the entire body are larger in patients with
liver disease because albumin and AGP concentrations are
lower in these patients and result in reduced lidocaine
plasma protein binding.
An index of liver dysfunction can be gained by applying the
Child-Pugh clinical classification system to the patient
Drug Interactions
Lidocaine has serious drug interactions with -
adrenergic receptor blockers and cimetidine that
decrease lidocaine clearance 30% or more.
Propranolol, metoprolol, and nadolol have been reported
to reduce lidocaine clearance due to the decrease in
cardiac output caused by -blocker agents. Decreased
cardiac output results in reduced liver blood flow which
explains the decline in lidocaine clearance caused by
these drugs.
Cimetidine also decreases lidocaine clearance, cimetidine
decreases lidocaine clearance by inhibiting hepatic
microsomal enzymes.
Lidocaine clearance may be accelerated by
concomitant use of phenobarbital or phenytoin.
Initial Dosage Determination
Methods
Pharmacokinetic Dosing Method
- Half-life and elimination rate costant estimate
- Volume of distribution estimate
- Selection of appropriate pharmacokinetic model and
equations
- Steady-state concentration.
Literature-based Recommended Dosing
Doses are based on those that commonly produce
steady-state concentrations in the lower end of the
therapeutic range, although there is a wide variation
in the actual concentrations for a specific patient.
USE OF LIDOCAINE BOOSTER DOSES TO
IMMEDIATELY
INCREASE SERUM CONCENTRATIONS
If a patient has a subtherapeutic lidocaine serum concentration and is
experiencing ventricular arrhythmias in an acute situation, it is desirable
to increase the lidocaine concentration as quickly as possible. In this
setting, it would not be acceptable to simply increase the maintenance
dose and wait 35 half-lives for therapeutic serum concentrations to be
established in the patient. A rational way to increase the serum
concentrations rapidly is to administer a booster dose of lidocaine, a
process also known as reloading the patient with lidocaine, computed
using pharmacokinetic techniques. A modified loading dose equation is
used to accomplish computation of the booster dose (BD) which takes into
account the current lidocaine concentration present in the patient:
BD = (C desired C actual )Vc,
C desired is the desired lidocaine concentration,
C is the actual current lidocaine concentration for the patient,
Vc is the central volume of distribution for lidocaine.
S-ar putea să vă placă și
- Digoxin PharmacokineticsDocument15 paginiDigoxin PharmacokineticsLama SaudÎncă nu există evaluări
- LidocaineDocument27 paginiLidocaineNadya PrafitaÎncă nu există evaluări
- Cardiovascular Agents FarklinDocument66 paginiCardiovascular Agents FarklinBundo NaqueÎncă nu există evaluări
- Lidocaine HydrochlorideDocument12 paginiLidocaine HydrochlorideShivam VinothÎncă nu există evaluări
- Putri RamadhanDocument44 paginiPutri RamadhanRama MulyadiÎncă nu există evaluări
- Antikonvulsan OkeDocument77 paginiAntikonvulsan OkeAci LusianaÎncă nu există evaluări
- ConclusionDocument1 paginăConclusionSamÎncă nu există evaluări
- DigoxinDocument2 paginiDigoxinFalaq2Încă nu există evaluări
- Management of Diabetic Ketoacidosis: MW SavageDocument3 paginiManagement of Diabetic Ketoacidosis: MW SavagedramaysinghÎncă nu există evaluări
- Antidyslipidemic Drugs (Geppetti)Document32 paginiAntidyslipidemic Drugs (Geppetti)Ariel OlshevskyÎncă nu există evaluări
- PDF DipiroDocument16 paginiPDF DipiroYolandaFandraIIÎncă nu există evaluări
- Anticoagulants by DR TariqDocument46 paginiAnticoagulants by DR Tariqsinan kÎncă nu există evaluări
- Approach ConsiderationsDocument5 paginiApproach ConsiderationsGunawan SetiawanÎncă nu există evaluări
- Endocrine Emergencies in The ICUDocument47 paginiEndocrine Emergencies in The ICUchadchimaÎncă nu există evaluări
- Acute Metabolic Complications of Diabetes MellitusDocument39 paginiAcute Metabolic Complications of Diabetes MellitusAli Murtaza AbbasÎncă nu există evaluări
- Heart Failure Dr. VishvasDocument31 paginiHeart Failure Dr. VishvasvishvasÎncă nu există evaluări
- Digoxin PharmacokineticDocument17 paginiDigoxin Pharmacokineticphd0780Încă nu există evaluări
- Digoxin ElixirDocument8 paginiDigoxin ElixirUlfha NurhamidahÎncă nu există evaluări
- سموم نظري٤Document6 paginiسموم نظري٤مصطفى ابراهيم سعيدÎncă nu există evaluări
- Xylocard PiDocument11 paginiXylocard PiRamakant SharmaÎncă nu există evaluări
- Management of Diabetic Ketoacidosis: MW SavageDocument3 paginiManagement of Diabetic Ketoacidosis: MW SavageChristian SalimÎncă nu există evaluări
- Management of Diabetic Ketoacidosis: A Summary of The 2013 Joint British Diabetes Societies GuidelinesDocument4 paginiManagement of Diabetic Ketoacidosis: A Summary of The 2013 Joint British Diabetes Societies GuidelinesErik Jaya GunawanÎncă nu există evaluări
- Drug Prescription in CKD and DialysisDocument24 paginiDrug Prescription in CKD and DialysisAnitha SÎncă nu există evaluări
- Use of Diuretics in CKDDocument19 paginiUse of Diuretics in CKDyulibudhyÎncă nu există evaluări
- Digoxin Immune Therapy: ComplicationsDocument15 paginiDigoxin Immune Therapy: ComplicationsDaryl Jacob BigayÎncă nu există evaluări
- PKVB Disease StateDocument39 paginiPKVB Disease StateDeepika ImmadiÎncă nu există evaluări
- TDM of LidocaineDocument19 paginiTDM of LidocaineNikkiiÎncă nu există evaluări
- DyslipidemiaDocument18 paginiDyslipidemiaDeepthi Avvaru100% (1)
- OB, Lactation, Pedi:: Route Onset Peak DurationDocument2 paginiOB, Lactation, Pedi:: Route Onset Peak Durationfryeabel1Încă nu există evaluări
- Vasoconstrictors & VasodilatorsDocument37 paginiVasoconstrictors & VasodilatorsDrVarun Menon100% (1)
- Welcome To Course Title: Clinical Pharmacy Topic: TDM of DigoxinDocument16 paginiWelcome To Course Title: Clinical Pharmacy Topic: TDM of DigoxinUmair MazharÎncă nu există evaluări
- Drug InteractionsDocument16 paginiDrug InteractionsYvetal GardeÎncă nu există evaluări
- Clinical Pharmacokinetics of PHENYTOINDocument69 paginiClinical Pharmacokinetics of PHENYTOINIndira ButkoonÎncă nu există evaluări
- EVMS Critical Care COVID-19 ProtocolDocument23 paginiEVMS Critical Care COVID-19 ProtocoldarwinÎncă nu există evaluări
- 6-Pharm Care Pada Terapi CairanDocument34 pagini6-Pharm Care Pada Terapi CairanApt RatnaningrumÎncă nu există evaluări
- Drug Round - DigoxinDocument4 paginiDrug Round - DigoxinErvinaDamayantiÎncă nu există evaluări
- 11) Drugs Used in DyslipidemiasDocument8 pagini11) Drugs Used in Dyslipidemiasفاعل خيرÎncă nu există evaluări
- Drug Induced Kidney DiseasesDocument34 paginiDrug Induced Kidney DiseasesManhal A AbdulkaderÎncă nu există evaluări
- Diagnosis and Treatment of Digoxin Toxicity PDFDocument3 paginiDiagnosis and Treatment of Digoxin Toxicity PDFRozi AbdullahÎncă nu există evaluări
- CHF 5Document2 paginiCHF 5Agus HaryantoÎncă nu există evaluări
- 6 - 2 - CVS Antiarrhythmic 2 by Col AzmatDocument72 pagini6 - 2 - CVS Antiarrhythmic 2 by Col AzmatAhmed YTÎncă nu există evaluări
- Obat Yang Bekerja Pada Sistem KardiovaskulerDocument18 paginiObat Yang Bekerja Pada Sistem KardiovaskulerMuhammad IkbalÎncă nu există evaluări
- Heart Failure DrugsDocument35 paginiHeart Failure Drugszmr27146Încă nu există evaluări
- LidocaineDocument4 paginiLidocaineFikrianisa SafrinaÎncă nu există evaluări
- Antidysrhythmic Drugs: Dr. SanoozDocument104 paginiAntidysrhythmic Drugs: Dr. SanoozsanoozarmÎncă nu există evaluări
- Drugs Acting On The Cardiovascular System-1Document28 paginiDrugs Acting On The Cardiovascular System-1gregorydonald315Încă nu există evaluări
- Midterms LecDocument276 paginiMidterms LecmyÎncă nu există evaluări
- Icu ProtocolDocument4 paginiIcu Protocolmehal guptaÎncă nu există evaluări
- HTN Treatment DR - VishvasDocument46 paginiHTN Treatment DR - VishvasvishvasÎncă nu există evaluări
- GUS4 Antihypertensive DrugsDocument7 paginiGUS4 Antihypertensive DrugsGabriella ChafrinaÎncă nu există evaluări
- Guidelines On: Acute Kidney InjuryDocument52 paginiGuidelines On: Acute Kidney InjuryWilsonne ChuaÎncă nu există evaluări
- Renal Replacement TherapyDocument46 paginiRenal Replacement TherapyPinky SahaÎncă nu există evaluări
- Challenges Diuretics CasebasedDocument13 paginiChallenges Diuretics CasebasedDaniel MelendezÎncă nu există evaluări
- AH PharmaII v2Document35 paginiAH PharmaII v2bankai2992Încă nu există evaluări
- Controlled Hypotension: Moderator: DR V. Y. Srinivas Presenter: Dr. Ann Susan MathewDocument49 paginiControlled Hypotension: Moderator: DR V. Y. Srinivas Presenter: Dr. Ann Susan MathewAnn Susan MathewÎncă nu există evaluări
- Aminoglycoside PharmacokineticsDocument13 paginiAminoglycoside PharmacokineticsLama SaudÎncă nu există evaluări
- Case Challenge: Diagnosing and Managing CKD Comorbidities: Literature ReviewDocument8 paginiCase Challenge: Diagnosing and Managing CKD Comorbidities: Literature ReviewPratik TripathiÎncă nu există evaluări
- 1Document9 pagini1herzi rahmatul sÎncă nu există evaluări
- 1Document9 pagini1herzi rahmatul sÎncă nu există evaluări
- Valproic AcidDocument19 paginiValproic Acidherzi rahmatul sÎncă nu există evaluări
- 2Document3 pagini2herzi rahmatul sÎncă nu există evaluări
- AparatusDocument15 paginiAparatusherzi rahmatul sÎncă nu există evaluări
- HEMODYALISISDocument8 paginiHEMODYALISISherzi rahmatul sÎncă nu există evaluări
- Medical PresipitationDocument16 paginiMedical Presipitationherzi rahmatul sÎncă nu există evaluări
- Medical PresipitationDocument16 paginiMedical Presipitationherzi rahmatul sÎncă nu există evaluări
- Orde 0Document6 paginiOrde 0herzi rahmatul sÎncă nu există evaluări
- Directly Proportional To Plasma Drug Concentration and Their Clearance Remains ConstantDocument6 paginiDirectly Proportional To Plasma Drug Concentration and Their Clearance Remains Constantherzi rahmatul sÎncă nu există evaluări
- DosisDocument8 paginiDosisherzi rahmatul sÎncă nu există evaluări
- Kinetika FixDocument13 paginiKinetika Fixherzi rahmatul sÎncă nu există evaluări
- Kinetika FixDocument13 paginiKinetika Fixherzi rahmatul sÎncă nu există evaluări
- Contact AngleDocument33 paginiContact Angleherzi rahmatul sÎncă nu există evaluări
- Soal Tast ToelfDocument9 paginiSoal Tast Toelfherzi rahmatul sÎncă nu există evaluări
- Soal Tast ToelfDocument9 paginiSoal Tast Toelfherzi rahmatul sÎncă nu există evaluări
- Previous Papers GPSC Veterinary Officer AHI Advt. No. 33 2016 17 Date of Preliminary Test 08 01 2017 Subject Concerned Subject Que 101 To 300 Provisional Key PDFDocument18 paginiPrevious Papers GPSC Veterinary Officer AHI Advt. No. 33 2016 17 Date of Preliminary Test 08 01 2017 Subject Concerned Subject Que 101 To 300 Provisional Key PDFDrRameem Bloch100% (1)
- NCPDocument6 paginiNCPJoni Lyn Ba-as BayengÎncă nu există evaluări
- Context in TranslationDocument23 paginiContext in TranslationRaluca FloreaÎncă nu există evaluări
- Science Grade 10 (Exam Prep)Document6 paginiScience Grade 10 (Exam Prep)Venice Solver100% (3)
- Course: Introduction To Geomatics (GLS411) Group Practical (2-3 Persons in A Group) Practical #3: Principle and Operation of A LevelDocument3 paginiCourse: Introduction To Geomatics (GLS411) Group Practical (2-3 Persons in A Group) Practical #3: Principle and Operation of A LevelalyafarzanaÎncă nu există evaluări
- Nuttall Gear CatalogDocument275 paginiNuttall Gear Catalogjose huertasÎncă nu există evaluări
- General Characteristics of Phonemes: Aspects of Speech SoundsDocument8 paginiGeneral Characteristics of Phonemes: Aspects of Speech SoundsElina EkimovaÎncă nu există evaluări
- CIGRE Operational Evaluation of RTV Coating Performance Over 17 Years On The Coastal Area at Jubail-SADocument9 paginiCIGRE Operational Evaluation of RTV Coating Performance Over 17 Years On The Coastal Area at Jubail-SAMalik Shoaib khalidÎncă nu există evaluări
- Ebook Stackoverflow For ItextDocument336 paginiEbook Stackoverflow For ItextAnonymous cZTeTlkag9Încă nu există evaluări
- Engine Stalls at Low RPM: Diagnostic CodesDocument3 paginiEngine Stalls at Low RPM: Diagnostic CodesAmir Bambang YudhoyonoÎncă nu există evaluări
- Market EquilibriumDocument36 paginiMarket EquilibriumLiraOhÎncă nu există evaluări
- 2012 Conference NewsfgfghsfghsfghDocument3 pagini2012 Conference NewsfgfghsfghsfghabdÎncă nu există evaluări
- Lecture 1 Electrolyte ImbalanceDocument15 paginiLecture 1 Electrolyte ImbalanceSajib Chandra RoyÎncă nu există evaluări
- Upadhyayaetal TrueliqtrigcurveDocument14 paginiUpadhyayaetal TrueliqtrigcurveVetriselvan ArumugamÎncă nu există evaluări
- NCP - DMDocument4 paginiNCP - DMMonica Garcia88% (8)
- Jordan CVDocument2 paginiJordan CVJordan Ryan SomnerÎncă nu există evaluări
- Offshore Training Matriz Matriz de Treinamentos OffshoreDocument2 paginiOffshore Training Matriz Matriz de Treinamentos OffshorecamiladiasmanoelÎncă nu există evaluări
- History of Drilling PDFDocument9 paginiHistory of Drilling PDFNguyen Van TinhÎncă nu există evaluări
- Model No. TH-65JX850M/MF Chassis. 9K56T: LED TelevisionDocument53 paginiModel No. TH-65JX850M/MF Chassis. 9K56T: LED TelevisionRavi ChandranÎncă nu există evaluări
- Reflective Memo 1-PracticumDocument5 paginiReflective Memo 1-Practicumapi-400515862Încă nu există evaluări
- Group 4&5 Activity Syntax AnalyzerDocument6 paginiGroup 4&5 Activity Syntax AnalyzerJuan PransiskoÎncă nu există evaluări
- Exercise-3 (B) : Linear EquationsDocument3 paginiExercise-3 (B) : Linear EquationsVRUSHABHÎncă nu există evaluări
- Java Edition Data Values - Official Minecraft WikiDocument140 paginiJava Edition Data Values - Official Minecraft WikiCristian Rene SuárezÎncă nu există evaluări
- OracleCarrierManifestingPartnerIntegration PDFDocument40 paginiOracleCarrierManifestingPartnerIntegration PDFvishal_vishnu11Încă nu există evaluări
- Teal Motor Co. Vs CFIDocument6 paginiTeal Motor Co. Vs CFIJL A H-DimaculanganÎncă nu există evaluări
- Most Probable Number (MPN) Test: Principle, Procedure, ResultsDocument4 paginiMost Probable Number (MPN) Test: Principle, Procedure, ResultsHammad KingÎncă nu există evaluări
- Phenomenology of The SelfDocument5 paginiPhenomenology of The SelfGuilherme CastelucciÎncă nu există evaluări
- CNC - Rdmacror: Public Static Extern Short Ushort Short Short ShortDocument3 paginiCNC - Rdmacror: Public Static Extern Short Ushort Short Short ShortKession HouÎncă nu există evaluări
- MMB & DFT 2012 Workshop ProceedingsDocument44 paginiMMB & DFT 2012 Workshop ProceedingsFelipe ToroÎncă nu există evaluări
- FM Testbank-Ch18Document9 paginiFM Testbank-Ch18David LarryÎncă nu există evaluări