Documente Academic
Documente Profesional
Documente Cultură
Bioremediation
Încărcat de
LaxusPlayz0 evaluări0% au considerat acest document util (0 voturi)
166 vizualizări53 paginiBioremediation uses microorganisms to degrade contaminants in water, soil, and other materials. It is often less expensive and more sustainable than alternative remediation methods. The microorganisms use contaminants and nutrients as electron acceptors and donors in redox reactions. Common bioremediation processes include aerobic biodegradation using oxygen, landfarming, composting, and bioventing. While many contaminants can be degraded, some heavy metals like cadmium and lead are more challenging for bioremediation.
Descriere originală:
Bioremediation Ppt
Drepturi de autor
© © All Rights Reserved
Formate disponibile
PPTX, PDF, TXT sau citiți online pe Scribd
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentBioremediation uses microorganisms to degrade contaminants in water, soil, and other materials. It is often less expensive and more sustainable than alternative remediation methods. The microorganisms use contaminants and nutrients as electron acceptors and donors in redox reactions. Common bioremediation processes include aerobic biodegradation using oxygen, landfarming, composting, and bioventing. While many contaminants can be degraded, some heavy metals like cadmium and lead are more challenging for bioremediation.
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PPTX, PDF, TXT sau citiți online pe Scribd
0 evaluări0% au considerat acest document util (0 voturi)
166 vizualizări53 paginiBioremediation
Încărcat de
LaxusPlayzBioremediation uses microorganisms to degrade contaminants in water, soil, and other materials. It is often less expensive and more sustainable than alternative remediation methods. The microorganisms use contaminants and nutrients as electron acceptors and donors in redox reactions. Common bioremediation processes include aerobic biodegradation using oxygen, landfarming, composting, and bioventing. While many contaminants can be degraded, some heavy metals like cadmium and lead are more challenging for bioremediation.
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PPTX, PDF, TXT sau citiți online pe Scribd
Sunteți pe pagina 1din 53
Bioremediation is a process used
to treat contaminated media, including
water, soil and subsurface material, by
altering environmental conditions to
stimulate growth of microorganisms and
degrade the target pollutants. In many
cases, bioremediation is less expensive
and more sustainable than other
remediation alternatives. Biological
treatment is a similar approach used to
treat wastes including wastewater,
industrial waste and solid waste.
Most bioremediation processes
involve oxidation-reduction (Redox)
reactions where a chemical species
donates an electron (electron donor) to
a different species that accepts the
electron (electron acceptor). During this
process, the electron donor is said to be
oxidized while the electron acceptor is
reduced. Common electron acceptors in
bioremediation processes include
oxygen, nitrate, manganese (III and IV),
iron (III), sulfate, carbon dioxide and
some pollutants (chlorinated solvents,
explosives, oxidized metals, and
radionuclides).
Electron donors include sugars, fats,
alcohols, natural organic material, fuel
hydrocarbons and a variety of reduced
organic pollutants. The redox potential for
common biotransformation reactions is
shown in the table.
In the event of biostimulation,
adding nutrients that are limited to make
the environment more suitable for
bioremediation, nutrients such as
nitrogen, phosphorus, oxygen, and
carbon may be added to the system to
improve effectiveness of the treatment.
Many biological processes are
sensitive to pH and function most
efficiently in near neutral conditions. Low
pH can interfere with pH homeostasis or
increase the solubility of toxic metals.
Microorganisms can expend cellular
energy to maintain homeostasis or
cytoplasmic conditions may change in
response to external changes in pH.
Some anaerobes have adapted to low pH
conditions through alterations in carbon
and electron flow, cellular morphology,
membrane structure, and protein
synthesis.
Aerobic bioremediation is the most
common form of oxidative bioremediation
process where oxygen is provided as the
electron acceptor for oxidation of
petroleum, polyaromatic hydrocarbons
(PAHs), phenols, and other reduced
pollutants. Oxygen is generally the
preferred electron acceptor because of
the higher energy yield and because
oxygen is required for some enzyme
systems to initiate the degradation
process.
Numerous laboratory and field
studies have shown that microorganisms
can degrade a wide variety of
hydrocarbons, including components of
gasoline, kerosene, diesel, and jet fuel.
Under ideal conditions, the
biodegradation rates of the low- to
moderate-weight aliphatic, alicyclic, and
aromatic compounds can be very high.
As the molecular weight of the compound
increases, so does the resistance to
biodegradation.
Common approaches for providing
oxygen above the water table include
landfarming, composting and bioventing.
During landfarming, contaminated soils,
sediments, or sludges are incorporated
into the soil surface and periodically
turned over (tilled) using conventional
agricultural equipment to aerate the
mixture. Composting accelerates
pollutant biodegradation by mixing the
waste to be treated with a bulking agent,
forming into piles, and periodically mixed
to increase oxygen transfer.
Bioventing is a process that
increases the oxygen or air flow into the
unsaturated zone of the soil which
increases the rate of natural in situ
degradation of the targeted hydrocarbon
contaminant.
Approaches for oxygen addition
below the water table include
recirculating aerated water through the
treatment zone, addition of pure oxygen
or peroxides, and air sparging.
Recirculation systems typically consist
of a combination of injection wells or
galleries and one or more recovery wells
where the extracted groundwater is
treated, oxygenated, amended with
nutrients and reinjected. However, the
amount of oxygen that can be provided
by this method is limited by the low
solubility of oxygen in water (8 to 10
mg/L for water in equilibrium with air at
typical temperatures).
Greater amounts of oxygen can be
provided by contacting the water with
pure oxygen or addition of hydrogen
peroxide (H2O2) to the water. In some
cases, slurries of solid calcium or
magnesium peroxide are injected under
pressure through soil borings. These
solid peroxides react with water
releasing H2O2 which then decomposes
releasing oxygen. Air sparging involves
the injection of air under pressure below
the water table. The air injection
pressure must be great enough to
overcome the hydrostatic pressure of the
water and resistance to air flow through
the soil.
According to the EPA,
Bioremediation is a “treatment that uses
naturally occurring organisms to break
down hazardous substances into less
toxic or non-toxic substances.”
1. It uses no chemicals
– One of the issues with using man-
made chemicals in the treatment and
removal of contamination is that the
chemicals eventually make it into the
water supply. There were many
chemicals used at the beginning of the
waste management era that we now
know were very harmful to plant, animal
and human life once they reached the
water supply.
2. It can allow waste to be recycled
– Another major reason that
bioremediation is preferred is that once
the waste is treated and the
contamination neutralized or removed,
the waste itself can then be recycled.
When chemical remediation types are
used, the waste is still contaminated just
with a less toxic substance and in
general, cannot then enter into the
recycle process. Bioremediation allows
for more waste to be recycled while
chemical methods still create waste that
cannot be used and has to be stored
somewhere.
• In-situ
– In situ refers to when
contaminated waste is treated right at its
point of origin. For example, there may be
soil that is contaminated. Rather than
remove the soil from its point of origin, it
is treated right where it is. The benefit to
in situ treatment is that it prevents the
spread of contamination during the
displacement and transport of the
contaminated material.
• Ex-situ
– Ex situ refers to treatment that
occurs after the contaminated waste has
been removed to a treatment area. To
use soil as the example again, the soil
may be removed and transported to an
area where the bioremediation may be
applied. The main advantage to this is it
helps to contain and control the
bioremediation products, as well as
making the area that was contaminated
available for use.
1. Phytoremediation
– use of plants to remove
contaminants. The plants are able to
draw the contaminants into their
structures and hold on to them,
effectively removing them from soil or
water.
2. Bioventing
– blowing air through soil to
increase oxygen rates in the waste. This
is an effective way to neutralize certain
oxygen sensitive metals or chemicals.
3. Bioleaching
– removing metals from soil using
living organisms. Certain types of
organisms are draw to heavy metals and
other contaminants and absorb them.
One new approach was discovered when
fish bones were found to attract and hold
heavy metals such as lead and cadmium.
4. Landfarming
– turning contaminated soil for
aeration and sifting to remove
contaminants, or deliberately depleting a
soil of nitrogen to remove nitrogen based
organisms.
5. Bioreactor
– the use of specially designed
containers to hold the waste while
bioremediation occurs.
6. Composting
– containing waste so a
natural decay and remediation
process occurs.
7. Bioaugmentation
– adding microbes and
organisms to strengthen the same
in waste to allow them to take over
and decontaminate the area.
8. Rhizofiltration
– the use of plants to remove
metals in water.
9. Biostimulation
– the use of microbes designed
to remove contamination applied in
a medium to the waste.
The major advantage of the
bioremediation methods is that it
allows for contamination to be
treated, neutralized or removed and
then produces a waste product
itself that is more easily disposed
of. In some cases, there is no need
for disposal at all. In the case of
the plants used in
phytoremediation and
rhizofiltration, the plant is able to
do something called
bioaccumulation.
This means is holds onto the
contaminant. As the plant is still
growing, there is no need to
remove and destroy it. In many
ways it is similar to having a
rechargeable battery. In the case
of contaminated waste, it is the
plant that keeps growing to allow
for more storage of waste. This is a
uniquely cost effective solution for
contaminated waste.
Below data has been taken from here
Microbial Population: Suitable kinds of
organisms that can biodegrade all of the
contaminants
Oxygen: Enough to support aerobic
biodegradation (about 2% oxygen in the
gas phase or 0.4 mg/liter in the soil
water)
Water: Soil moisture should be from 50–
70% of the water holding capacity of the
soil
Nutrients: Nitrogen, phosphorus, sulfur,
and other nutrients to support good
microbial growth
Temperature: Appropriate temperatures
for microbial growth (0–40˚C)
pH: Best range is from 6.5 to 7.5
There are some types of contamination
that are very difficult to use bioremediation
for. The two biggest concerns are:
1. Cadmium
2. Lead
Both of these are classified as heavy
metals and are difficult to remove using
microorganisms. As mentioned earlier, a
recent discovery about the absorption rate of
fish bone has proving successful In fact,
bone seems to hold the clue for removing
heavy metal contamination. Char is used to
remove small amounts of zinc, lead and
cadmium; and it is thought that the calcium
in the fish bone is what makes it effective .
Can you use Bioremediation on Nuclear Waste?
Yes and no. While you can’t apply any
microorganism to nuclear waste with any great
success, two bioremediation techniques are used
to handle nuclear waste.
1. Bioreactor
– Nuclear waste is already contained
within vessels that prevent the contamination
from spreading. This becomes a bioremediation
because of what then happens within that
container.
2. Composting
– When you think about it, nuclear waste
goes through the exact same process as material
you place in a composting pile. The waste has
everything it needs to break itself down, it just
takes much longer. Once it is secured in the
bioreactor vessel, the naturally occurring process
of bio remediation takes over.
Part of the conservation efforts by
scientists are focusing on the effective
use of bioremediation on contaminated
waste. Contaminated waste comes from
two different causes –
1. Natural
2. Man-made
Bioremediation was invented by
George M. Robinson in the 1960s. Robinson,
working as the assistant county engineer for
Santa Maria, California, organized the first
large-scale microbial cleanup of an oil spill in
1968. Robinson used bioremediation to
complete the clean up of spills, sewage,
leach fields as well as odor and pest control.
Today, microbes are used to treat sewage,
oil spills, contaminated soil and increase
yields in food production. Nearly every
company that competes in this market place
uses bug cultures that can be traced back to
George Robinson or one of his colleagues.
Bioremediation has been used in
several famous cleanups such as the
Exxon Valdez Oil Spill in Alaska in 1989.
Alongside the many volunteers who
worked to clean the 11 million gallons of
spilled oil, microbes worked with them by
breaking down oil as their food source.
By applying specific microbes to a
pollutant spill, engineers can help to
speed up the process, minimizing the
damage to the environment.
The U.S. Geological Survey has had
tremendous success with
bioremediation. Their practice of using
bioremediation has contributed to the
safe, effective cleanup of many spills,
some dangerously toxic, as well as the
advancement of bioremediation
knowledge and know-how. Some of their
successes are listed below.
• Crude oil spill, Bemidji, Minnesota: In
1979, a pipeline carrying crude oil burst and
contaminated the underlying aquifer. USGS
scientists studying the site found that toxic
chemicals leaching from the crude oil were
rapidly degraded by natural microbial
populations. Significantly, it was shown that
the plume of contaminated ground water
stopped enlarging after a few years as rates
of microbial degradation came into balance
with rates of contaminant leaching. This was
the first and best-documented example of
intrinsic bioremediation in which naturally
occurring microbial processes remediates
contaminated ground water without human
intervention.
• Sewage effluent, Cape Cod,
Massachusetts: Disposal of sewage
effluent in septic drain fields is a
common practice throughout the United
States. Systematic studies of a sewage
effluent plume at Massachusetts Military
Reservation (formerly known as Otis Air
Force Base) led to the first accurate field
and laboratory measurements of how
rapidly natural microbial populations
degrade nitrate contamination
(denitrification) in a shallow aquifer.
• Chlorinated solvents, New Jersey:
Chlorinated solvents are a particularly
common contaminant in the heavily-
industrialized Northeast. Because their
metabolic processes are so adaptable,
microorganisms can use chlorinated
compounds as oxidants when other
oxidants are not available. Such
transformations, which can naturally
remediate solvent contamination of
ground water, have been extensively
documented by USGS scientists at
Picatinny Arsenal, New Jersey.
• Pesticides, San Francisco Bay
Estuary: Pesticide contamination of
rivers and streams is a matter of concern
throughout the United States. Field and
laboratory studies in the Sacramento
River and San Francisco Bay have shown
the effects of biological and non-
biological processes in degrading
commonly used pesticides, such as
molinate, thiobencarb, carbofuran and
methyl parathion.
• Agricultural chemicals in the
midcontinent: Agricultural chemicals
affect the chemical quality of ground
water in many Midwestern States.
Studies in the midcontinent have traced
the fate of nitrogen fertilizers and
pesticides in ground and surface waters.
These studies have shown that many
common contaminants, such as the
herbicide atrazine, are degraded by
biological (microbial degradation) and
non-biological (photolytic degradation)
processes.
• Gasoline contamination, Galloway,
New Jersey: Gasoline is probably the
most common contaminant of ground
water in the United States. Studies at
this site have demonstrated rapid
microbial degradation of gasoline
contaminants and have shown the
importance of processes in the
unsaturated zone (the zone above the
water table) in degrading contaminants.
1.What process used to treat contaminated
media, including water, soil and subsurface
material?
2.What is the short term for oxidation reduction?
3.It is the most common form of oxidative
bioremediation process.
4. Give 1 importance of bioremediation
5. What class of bioremediation refers to
treatment that occurs after the contaminated
waste has been removed to a treatment area?
6. It the use of plants to remove metals in water.
7. What type of bioremediation blows the air
through soil to increase oxygen rates in the
waste?
8.9. Give the 2 types of contamination that are
very difficult to use bioremediation for.
10. ______________ has had tremendous success
with bioremediation.
1. Bioremediation
2. Redox
3. Aerobic bioremediation
4. -It uses no chemicals
-It can allow waste to be
recycled
5. Ex-situ
6. Rhizofiltration
7. Bioventing
8. Cadmium/Lead
9. Cadmium/Lead
10.U.S. Geological Survey
Ma. Amberainne P. Mendoza
Eunice Anne P. Dela Cruz
Karl Angelo Miguel C. Roque
Mrs. Lucena S. Paguinto
S-ar putea să vă placă și
- Bioremediation: Resna N K Assistant Professor Gems CollegeDocument40 paginiBioremediation: Resna N K Assistant Professor Gems CollegeResna N K ResiÎncă nu există evaluări
- Bioremediation: How Does Bioremediation WorksDocument4 paginiBioremediation: How Does Bioremediation WorksswtoÎncă nu există evaluări
- Wk2 Bioremediation and Bio DegradationDocument52 paginiWk2 Bioremediation and Bio DegradationraitavÎncă nu există evaluări
- BioremediationDocument10 paginiBioremediationArush SidanaÎncă nu există evaluări
- Final Bioremediation - PicsDocument68 paginiFinal Bioremediation - PicsKawish BhattiÎncă nu există evaluări
- Bio RemediationDocument24 paginiBio RemediationMinhaj HaiderÎncă nu există evaluări
- Polluted Environment Bioremediation ofDocument5 paginiPolluted Environment Bioremediation ofrockingtwo07Încă nu există evaluări
- Effluent Treatment2Document14 paginiEffluent Treatment2Mirza Md. Nazmus SakibÎncă nu există evaluări
- Microbes in Sewage TreatmentDocument11 paginiMicrobes in Sewage Treatmentyukti86% (36)
- Definition: Use of Living: Organisms To Transform, Destroy or Immobilize ContaminantsDocument22 paginiDefinition: Use of Living: Organisms To Transform, Destroy or Immobilize ContaminantsDivya DiyaÎncă nu există evaluări
- EnviorDocument9 paginiEnviorAYESHA ALIÎncă nu există evaluări
- Development of Alternate Cleaner Technologies Using BiotechnologyDocument6 paginiDevelopment of Alternate Cleaner Technologies Using BiotechnologyNeeraj AnsalÎncă nu există evaluări
- Introduction To BiotechnologyDocument4 paginiIntroduction To BiotechnologyyounusjugnoÎncă nu există evaluări
- BIOREMEDIATIONDocument31 paginiBIOREMEDIATIONTU_MTECH_ENV11Încă nu există evaluări
- Wastewater Disposal.Document266 paginiWastewater Disposal.FarazÎncă nu există evaluări
- Biotech Handout LAS 4.2Document30 paginiBiotech Handout LAS 4.2Denise LautaÎncă nu există evaluări
- Lecture - 3 - Wastewater Treatment - Aerobic and Anaerobic-1Document11 paginiLecture - 3 - Wastewater Treatment - Aerobic and Anaerobic-1negaathanipalanisamyÎncă nu există evaluări
- The American University in Cairo: Environmental Science Water Pollution Wastewater TreatmentDocument43 paginiThe American University in Cairo: Environmental Science Water Pollution Wastewater TreatmentBabbooÎncă nu există evaluări
- Removal of Organic Dissolved SolidsDocument21 paginiRemoval of Organic Dissolved SolidsShravik Patni 18BCL0040Încă nu există evaluări
- Microbes in Human WelfareDocument17 paginiMicrobes in Human WelfareArchana BÎncă nu există evaluări
- Decomposition of Organic Matter in WaterDocument6 paginiDecomposition of Organic Matter in WaterDivya Reddy100% (1)
- Wastewater Treatment Mini ReviewDocument26 paginiWastewater Treatment Mini Reviewnininick805Încă nu există evaluări
- Bioremediation: Jump To Navigation Jump To SearchDocument6 paginiBioremediation: Jump To Navigation Jump To SearchDharmendra SinghÎncă nu există evaluări
- "Remediate": Means To Solve A ProblemDocument9 pagini"Remediate": Means To Solve A ProblemNiko ChubongÎncă nu există evaluări
- A General Essay On Bioremediation of Contaminated SoilDocument5 paginiA General Essay On Bioremediation of Contaminated SoildianpalulinaÎncă nu există evaluări
- Water TreatementDocument7 paginiWater TreatementAnuj KumarÎncă nu există evaluări
- Biotechnological Methods For ManagementDocument30 paginiBiotechnological Methods For ManagementValeska CherylÎncă nu există evaluări
- Biodegradation NewDocument37 paginiBiodegradation NewAyesha AbbasiÎncă nu există evaluări
- Waste WaterDocument93 paginiWaste Watersobhan hamidipourÎncă nu există evaluări
- What Is BioremediationDocument8 paginiWhat Is BioremediationAbdulkadir AlbabaÎncă nu există evaluări
- Microbes Are Omnipresent in The BiosphereDocument9 paginiMicrobes Are Omnipresent in The BiosphereM A RazzakÎncă nu există evaluări
- Waste Water Treatment ProcessDocument4 paginiWaste Water Treatment ProcessDengAwutÎncă nu există evaluări
- Gee - V UnitDocument12 paginiGee - V UnitPvÎncă nu există evaluări
- Remediation of Contaminated SoilDocument55 paginiRemediation of Contaminated SoilAhmedAmer1100% (1)
- BIOREMEDIATION GoodDocument8 paginiBIOREMEDIATION GoodAmiruddin AliÎncă nu există evaluări
- Pseudomonas in Biodegradation PDFDocument10 paginiPseudomonas in Biodegradation PDFPenelope MeloÎncă nu există evaluări
- Lecture Iii Cve 503 Biological Treatment of Wastewater1Document41 paginiLecture Iii Cve 503 Biological Treatment of Wastewater1James Korbla Dodzi Hills100% (1)
- Microbiology of WastewaterDocument5 paginiMicrobiology of WastewaterAnupriyaÎncă nu există evaluări
- Pli B3Document50 paginiPli B3Mahendra PuguhÎncă nu există evaluări
- Biological Treatment Solid Hazardous WasteDocument18 paginiBiological Treatment Solid Hazardous WasteLalthakima PachuauÎncă nu există evaluări
- Jimma University Water Resource and Environmental Engineering (Wree)Document6 paginiJimma University Water Resource and Environmental Engineering (Wree)Temesgen DemessieÎncă nu există evaluări
- Fermentation Technology in Sewage Waste WaterDocument4 paginiFermentation Technology in Sewage Waste Waterfama18Încă nu există evaluări
- I. Nature and Types of Water Pollutants: A) Petroleum ProductsDocument16 paginiI. Nature and Types of Water Pollutants: A) Petroleum ProductsberlynforscribdÎncă nu există evaluări
- Bioremediation: Rohit Chandak 1MS08CH038Document20 paginiBioremediation: Rohit Chandak 1MS08CH038Rohit ChandakÎncă nu există evaluări
- Lecture Iv Cve 503 Biological Treatment of WastewaterDocument43 paginiLecture Iv Cve 503 Biological Treatment of WastewaterJames Korbla Dodzi HillsÎncă nu există evaluări
- Biotech Environ. Biotech. 1Document12 paginiBiotech Environ. Biotech. 1saleemÎncă nu există evaluări
- Topic 5 NotesDocument11 paginiTopic 5 Notesapi-293573854Încă nu există evaluări
- Ce 1304 Environmental EngineeringDocument44 paginiCe 1304 Environmental EngineeringprashmceÎncă nu există evaluări
- Reaction Paper - Water Waster Characteristics and Sewage TreatmentDocument2 paginiReaction Paper - Water Waster Characteristics and Sewage TreatmentChilton John DuatÎncă nu există evaluări
- Intro Evn - BiotechDocument12 paginiIntro Evn - BiotechMuhammad Mudassar ChudaryÎncă nu există evaluări
- General Design ConsiderationDocument17 paginiGeneral Design ConsiderationOwaisÎncă nu există evaluări
- Waste Water TreatmentDocument10 paginiWaste Water TreatmentPriyank LashkariÎncă nu există evaluări
- Environmental Microbiology, Sewage Treatment and Industrial Microbiology DR Mark AshbyDocument29 paginiEnvironmental Microbiology, Sewage Treatment and Industrial Microbiology DR Mark Ashbydanena88Încă nu există evaluări
- Use of Biotechnology For Cleaning Up Our Environment: Landfill TechnologiesDocument12 paginiUse of Biotechnology For Cleaning Up Our Environment: Landfill TechnologiesJoyce Ann LinglingÎncă nu există evaluări
- Wikipedia BioremediationDocument3 paginiWikipedia BioremediationPanayiotis KatsaberisÎncă nu există evaluări
- Lec.1&2 Environ. 2024Document52 paginiLec.1&2 Environ. 2024Nourhan SobhyÎncă nu există evaluări
- Water PollutionDocument9 paginiWater PollutionDAYA RAM SAHÎncă nu există evaluări
- Sanity SeminarDocument8 paginiSanity Seminarsani sundayÎncă nu există evaluări
- Microbiological Aspects of Pollution Control: Fundamental Aspects of Pollution Control and Environmental ScienceDe la EverandMicrobiological Aspects of Pollution Control: Fundamental Aspects of Pollution Control and Environmental ScienceÎncă nu există evaluări
- Vegetable Production in Greenhouses: greenhouse Production, #3De la EverandVegetable Production in Greenhouses: greenhouse Production, #3Încă nu există evaluări
- Research Plan Xylaria HongkongensisDocument21 paginiResearch Plan Xylaria HongkongensisLaxusPlayzÎncă nu există evaluări
- CC Smoking Fish 1Document1 paginăCC Smoking Fish 1LaxusPlayzÎncă nu există evaluări
- Arduino Uno-Based Water Turbidity Meter Using LDR and LED SensorsDocument6 paginiArduino Uno-Based Water Turbidity Meter Using LDR and LED SensorsLaxusPlayzÎncă nu există evaluări
- English StoryDocument2 paginiEnglish StoryLaxusPlayzÎncă nu există evaluări
- SagingDocument3 paginiSagingLaxusPlayzÎncă nu există evaluări
- Main Idea WorksheetDocument1 paginăMain Idea WorksheetLaxusPlayzÎncă nu există evaluări
- Golden Riceu 1Document22 paginiGolden Riceu 1LaxusPlayzÎncă nu există evaluări
- Bee Keeping-KVK MorenaDocument6 paginiBee Keeping-KVK MorenaAsh1Scribd100% (1)
- Nephrotic SyndromeDocument56 paginiNephrotic SyndromeMurugesan100% (1)
- MCS120 220 Error Ref - GAA30082DAC - RefDocument21 paginiMCS120 220 Error Ref - GAA30082DAC - RefCoil98Încă nu există evaluări
- What Does She/He Look Like?: Height Build AGEDocument18 paginiWhat Does She/He Look Like?: Height Build AGEHenrich Garcia LimaÎncă nu există evaluări
- ISCO HDPE Full Line CatalogDocument252 paginiISCO HDPE Full Line Catalogpvsreddy2002100% (1)
- Ethics, Privacy, and Security: Lesson 14Document16 paginiEthics, Privacy, and Security: Lesson 14Jennifer Ledesma-Pido100% (1)
- Uni of Glasgow Map PDFDocument1 paginăUni of Glasgow Map PDFJiaying SimÎncă nu există evaluări
- RCMaDocument18 paginiRCMaAnonymous ffje1rpaÎncă nu există evaluări
- Musa Paradisiaca L. and Musa Sapientum L.: A Phytochemical and Pharmacological ReviewDocument8 paginiMusa Paradisiaca L. and Musa Sapientum L.: A Phytochemical and Pharmacological ReviewDeviÎncă nu există evaluări
- Marketing ProjectDocument82 paginiMarketing ProjectSumit GuptaÎncă nu există evaluări
- Nursing Care Plan: Assessment Diagnosis Planning Interventions Rationale EvaluationDocument11 paginiNursing Care Plan: Assessment Diagnosis Planning Interventions Rationale EvaluationDa NicaÎncă nu există evaluări
- Cuts of BeefDocument4 paginiCuts of BeefChristopher EnriquezÎncă nu există evaluări
- NASA ISS Expedition 2 Press KitDocument27 paginiNASA ISS Expedition 2 Press KitOrion2015Încă nu există evaluări
- Theories of Learning and Learning MetaphorsDocument4 paginiTheories of Learning and Learning MetaphorsTrisha Mei Nagal50% (2)
- Main CatalogueDocument12 paginiMain Catalogueferpa_ferÎncă nu există evaluări
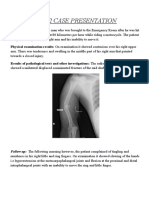
- PBL 2 Case PresentationDocument12 paginiPBL 2 Case PresentationRamish IrfanÎncă nu există evaluări
- Preservative MaterialsDocument2 paginiPreservative MaterialsmtcengineeringÎncă nu există evaluări
- Oral Rehydration SolutionDocument22 paginiOral Rehydration SolutionAlkaÎncă nu există evaluări
- 5070 s14 QP 11Document16 pagini5070 s14 QP 11OsamaRahimÎncă nu există evaluări
- OphthalmoplegiaDocument5 paginiOphthalmoplegiaPatricia Feliani SitohangÎncă nu există evaluări
- Dermatology Mini-OSCEDocument322 paginiDermatology Mini-OSCEMarrkÎncă nu există evaluări
- Containers HandbookDocument26 paginiContainers Handbookrishi vohraÎncă nu există evaluări
- Top 6 Beginner Work Out MistakesDocument4 paginiTop 6 Beginner Work Out MistakesMARYAM GULÎncă nu există evaluări
- Occlusal Appliance TherapyDocument14 paginiOcclusal Appliance TherapyNam BuiÎncă nu există evaluări
- طبى 145Document2 paginiطبى 145Yazan AbuFarhaÎncă nu există evaluări
- We Find The Way: Shipping InstructionsDocument10 paginiWe Find The Way: Shipping InstructionsLuke WangÎncă nu există evaluări
- Research Activity #2Document2 paginiResearch Activity #2Shania GualbertoÎncă nu există evaluări
- Pure Vegeterian: Kousika (CaterersDocument2 paginiPure Vegeterian: Kousika (CaterersShylender NagaÎncă nu există evaluări
- Additional Activity 3 InsciDocument3 paginiAdditional Activity 3 InsciZophia Bianca BaguioÎncă nu există evaluări
- Om Deutz 1013 PDFDocument104 paginiOm Deutz 1013 PDFEbrahim Sabouri100% (1)