Documente Academic
Documente Profesional
Documente Cultură
Module 4b - Stainless Steels
Încărcat de
Anonymous 7yN43wjlDescriere originală:
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Module 4b - Stainless Steels
Încărcat de
Anonymous 7yN43wjlDrepturi de autor:
Formate disponibile
METALLURGY DEPARTMENT
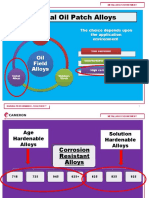
Typical Oil Patch Alloys
The choice depends upon
Carbon the application
Steels
environment
Low corrosion
Oil Intermediate corrosion
Field
Alloys High corrosion
Nickel Stainless
Alloys Steels
RAISING PERFORMANCE. TOGETHERTM 1
METALLURGY DEPARTMENT
What makes stainless steels
corrosion resistant?
Thin Oxide
Stainless Steels
Steels Layer
Fe-base; Cr > 10%
Duplex/su 17-4PH
316 414 F6NM per
duplex
Base Metal What corrodes?: the Fe base metal
corrodes in the presence of air, so
a thin Cr-oxide layer eats up the
oxygen and prevents any
penetrating further in.
RAISING PERFORMANCE. TOGETHERTM 2
METALLURGY DEPARTMENT
Typical Stainless Steels
Stainless
Except
steelsfor
Grade have
Most316,
C a
ofthe
thecontent
stainless
Mn alloys
Cr Ni Mo
high content
of nickel
of Cr have
above
is generally
low molylow
contents.
Except for the martensitic
10% & for the
Austenitic 316duplex0.08
alloys
Low moly2.00implies low
16.0- the10.0-
stainless; carbon 2.0-
above 20% Max pitting resistance.
Max content
18.0 is generally
14.0 very 3.0
low.
Ferritic 430 0.12 1.00 16.0- - -
Max Max 18.0
Martensitic 410 0.15 1.00 11.5- - -
Max Max 13.5
Duplex 2205 0.03 2.00 21.0- 4.5-6.5 2.5-
Max Max 23.0 3.5
Precipitation 17-4 0.07 1.00 15.0- 3.00- -
Hardenable Max Max 17.5 5.00
RAISING PERFORMANCE. TOGETHERTM 3
METALLURGY DEPARTMENT
Chemistries define The Structure of
Stainless Alloys
Ni
Ni equivalents or g-stabilizer Mn
FCC crystal structure Co
Cr
Cr equivalents or a-stabilizer Si
BCC crystal structure Mo
Nb
RAISING PERFORMANCE. TOGETHERTM 4
METALLURGY DEPARTMENT
Compare these two stainless alloy
compositions
316L Stainless
410 Stainless steel Steel
Element %’age Element %’age
C .1 C .03
Cr 12.5 Cr 18
Ni - Ni 12
Mo - Mo 3
Balance Fe Balance Fe
a-stabilizers
martensitic gaustenitic
-stabilizers
Go into solution in the BCC Go into solution in the FCC
crystal structure crystal structure
RAISING PERFORMANCE. TOGETHERTM 5
METALLURGY DEPARTMENT
Relationship
Consider abetween composition
duplex stainless steeland
structure
Why is (Schaeffler diagram)
it called duplex?
Grade C Mn Cr Ni Mo
Creq = %Cr + 1.5 X %Si + %Mo
Duplex 2205 0.03 2.00 21.0- 4.5-6.5 2.5-
Nieq = %Ni + 30(%C+%N)
Max + 0.5Max
X (%Mn 23.0
+ %Cu + %Co) 3.5
Creq = %Cr + 1.5 X %Si + %Mo = 26.5 316
Stainless
Nieq = %Ni + 30(%C+%N) + 0.5 X (%Mn + %CuSteel
+ %Co) = 6.5
(18%Cr & 12% NI)
Austenite
Ferrite
410
Stainless Steel
(12%Cr & 0% NI)
RAISING PERFORMANCE. TOGETHERTM 6
METALLURGY DEPARTMENT
Pitting Corrosion a concern when
dealing with Stainless Steels
Pitting occurs when surface of stainless is damaged
subsea. Passive layer cannot reform.
The positively
charged pits
attract the
chlorine ions that
can combine with
the (OH) to form
HCL or
hydrochloric acid.
RAISING PERFORMANCE. TOGETHERTM 7
METALLURGY DEPARTMENT
PREN %Cr + 3.3(%Mo) + 30(%N)
Grade Cr Mo N PREN
304 Stainless
steel cannot be
Austenitics used subsea
dueStainless
316 to poor
pitting
can only be
304 18.5 - .11
resistance
used max
subsea 18.5
with CP
protection
316 18 2 .11 max 22
Duplex
can only be
Duplex used subsea
Super
with Duplex
CP
Can be used
protection
2205 22 3 .22
subsea without 35
CP protection
2507 25 3.5 .25 >40
RAISING PERFORMANCE. TOGETHERTM 8
METALLURGY DEPARTMENT
How does Mo, Cr & N increase pitting corrosion
resistance?
Molybdate ions are oxides of molybdenum.
These form on the surface of the pit and can act
to raise the ph in that are oounter acting a and
hence halt corrosion by allowing the
reformation of the Cr oxide film.
M
M
o
o M M N
N
M M
o M
M o
o M
M o
o
o
o
o
Acidic conditions in the pit activate
corrosion & the loss of iron ions
RAISING PERFORMANCE. TOGETHERTM 9
METALLURGY DEPARTMENT
Case Study: Noble Aseng 2011 SCM duplex control lines
leaking after pressure tests
Note ferrite structure in weld & duplex
Cross section across weld shows
Note hydraulic austenite/ferrite structure in base metal.
Autogenous welding is done withoutcracks
filler metal
which were using
source ofonly
leaks the TIG torch to join two tubing
control line tubing
made out of super ends. Ferrite
SCM activates
duplex stainless hydraulics in Trees.
steel. If there is leak, the
equipment is Duplex
essentially paralyzed.
Contrast to the what the weld/base metal structure
High heat
should
input at low speeds stabilizes look like
under standard circumstances. ferritic phase
Control line tubing
is bent & welded to
in various places as
shown. Leaking
was found to occur
at these weld
joints.
Cause of failure was welding procedure
which stabilized ferritic phase which is
susceptible to HISC.
RAISING PERFORMANCE. TOGETHERTM 10
METALLURGY DEPARTMENT
Common Issues with Stainless
Steels
Sigma (σ)
Sensitization (M23C6)
RAISING PERFORMANCE. TOGETHERTM 11
S-ar putea să vă placă și
- Taxonomy of MetalsDocument28 paginiTaxonomy of MetalsArlita RahmaÎncă nu există evaluări
- DESIGN 1 NotesDocument7 paginiDESIGN 1 NoteslordyÎncă nu există evaluări
- WHY STUDY Applications and Processing of Metal Alloy?Document24 paginiWHY STUDY Applications and Processing of Metal Alloy?hanizznabÎncă nu există evaluări
- Module 4c - Corrosion Resistant Alloys (CRA's)Document11 paginiModule 4c - Corrosion Resistant Alloys (CRA's)Anonymous 7yN43wjlÎncă nu există evaluări
- Alloying ElementsDocument51 paginiAlloying Elements庄查理Încă nu există evaluări
- 7 - Wrap Up Session For Mid Term TestDocument36 pagini7 - Wrap Up Session For Mid Term TestFiky ArdiansyahÎncă nu există evaluări
- Applications and Processing of Metal AlloysDocument39 paginiApplications and Processing of Metal Alloysjulito paquitÎncă nu există evaluări
- Intergranular CorrosionDocument20 paginiIntergranular CorrosionD'yana RusdiÎncă nu există evaluări
- Material InspectionDocument39 paginiMaterial Inspectionkrisman f siregarÎncă nu există evaluări
- CH 11Document72 paginiCH 11Paolo SumaldeÎncă nu există evaluări
- LM6 Casting Alloy PDFDocument2 paginiLM6 Casting Alloy PDFSankarÎncă nu există evaluări
- Operational Information Bearing MaterialsDocument2 paginiOperational Information Bearing MaterialsHim SatiÎncă nu există evaluări
- Microstructures of Iron-Carbon Alloys: Fine Pearlite 3000XDocument9 paginiMicrostructures of Iron-Carbon Alloys: Fine Pearlite 3000XVaishu 07Încă nu există evaluări
- Stainless SteelDocument4 paginiStainless SteelJoemar AnchetaÎncă nu există evaluări
- Chapter 11Document52 paginiChapter 11TamiruÎncă nu există evaluări
- Stainless SteelDocument56 paginiStainless SteelShariq KhanÎncă nu există evaluări
- Chapter 1 (A) Corrosion SlideDocument26 paginiChapter 1 (A) Corrosion SlideNasyitah TarmiziÎncă nu există evaluări
- Application and Processing of Metal AlloysDocument44 paginiApplication and Processing of Metal AlloysShaira DaleÎncă nu există evaluări
- Vi. Ceramics and MetalDocument4 paginiVi. Ceramics and MetalLudwig RamosÎncă nu există evaluări
- Day 2 Part 1Document90 paginiDay 2 Part 1Edukondalu PentapatiÎncă nu există evaluări
- Material - (1 4462)Document2 paginiMaterial - (1 4462)Ashutosh PathakÎncă nu există evaluări
- Ch13 Materials ApplicationsDocument63 paginiCh13 Materials ApplicationsThefairman UnkownÎncă nu există evaluări
- Ch13 Materials ApplicationsDocument69 paginiCh13 Materials ApplicationsRhanganath ArivudainambiÎncă nu există evaluări
- Metal - Designation & PropertiesDocument37 paginiMetal - Designation & Propertiesmyself_riteshÎncă nu există evaluări
- UNIT 3 Ferrous and Non Ferrous MetalsDocument68 paginiUNIT 3 Ferrous and Non Ferrous MetalsAmutha PSGRKCWÎncă nu există evaluări
- Chapter 11: Metal Alloys Applications and Processing: Issues To Address..Document21 paginiChapter 11: Metal Alloys Applications and Processing: Issues To Address..Naufal PutraÎncă nu există evaluări
- Issues To Address... : Chapter 11 - 1Document20 paginiIssues To Address... : Chapter 11 - 1Rowen PratherÎncă nu există evaluări
- Balachandar - Cast & Duplex SSDocument155 paginiBalachandar - Cast & Duplex SSArunprasad MurugesanÎncă nu există evaluări
- Various Categories of Stainless Steels Produced in The Country and Characteristics August 2021Document59 paginiVarious Categories of Stainless Steels Produced in The Country and Characteristics August 2021Adrija DeÎncă nu există evaluări
- GTAW Welding of CP Titanium and Ti6Al4VDocument1 paginăGTAW Welding of CP Titanium and Ti6Al4VDeepak KumarÎncă nu există evaluări
- ch11 - 2metal Alloys Application and ProcessingDocument75 paginich11 - 2metal Alloys Application and ProcessingZuhaÎncă nu există evaluări
- Chapter 11 Metal AlloysDocument24 paginiChapter 11 Metal Alloyssihar raymondÎncă nu există evaluări
- Baja Karbon (Carbon Steels)Document25 paginiBaja Karbon (Carbon Steels)Mochammad Fajri MuharamÎncă nu există evaluări
- Iron and SteelDocument22 paginiIron and SteelAmarendra Hassan100% (3)
- Aluminium Alloy 2014A T651 Sheet and Plate: Specifications Temper TypesDocument3 paginiAluminium Alloy 2014A T651 Sheet and Plate: Specifications Temper TypesBeni hidayatullahÎncă nu există evaluări
- Fmatsci PDFDocument2 paginiFmatsci PDFGenesis Tolentino GonzalesÎncă nu există evaluări
- Module 4 and 6: Luckman MuhmoodDocument54 paginiModule 4 and 6: Luckman MuhmoodAman PanchalÎncă nu există evaluări
- Steel PropertiesDocument22 paginiSteel PropertiesMOHD SALMANÎncă nu există evaluări
- Key Facts Typical Wire Analysis: - Bossweld 316LSIDocument1 paginăKey Facts Typical Wire Analysis: - Bossweld 316LSIAli TalebiÎncă nu există evaluări
- Steels Used Onboard Ships and How To Perform Maintenance WeldingDocument35 paginiSteels Used Onboard Ships and How To Perform Maintenance Weldingantonio111aÎncă nu există evaluări
- DSMTS-0010.6 ZN WireDocument4 paginiDSMTS-0010.6 ZN WireMario Cortes FariasÎncă nu există evaluări
- Joining Stainless SteelDocument4 paginiJoining Stainless SteelPuneet KankariaÎncă nu există evaluări
- Refinery & CastingDocument43 paginiRefinery & CastingMuhammad Sandi ArifÎncă nu există evaluări
- Lect 9. Steel Classification and PropertiesDocument28 paginiLect 9. Steel Classification and PropertiesRio BuiÎncă nu există evaluări
- Part 4 Nonferrous AlloysDocument23 paginiPart 4 Nonferrous AlloysAhmed awwadÎncă nu există evaluări
- Structural Stainless Steel Designing With Stainless Steel: Ing. Maarten FortanDocument153 paginiStructural Stainless Steel Designing With Stainless Steel: Ing. Maarten FortanJohn Philip Neri BesedillasÎncă nu există evaluări
- Alloy SteelsDocument31 paginiAlloy Steelsdawitdafe4Încă nu există evaluări
- NL - NL FactSheet - Main 01Document1 paginăNL - NL FactSheet - Main 01ronaldb322Încă nu există evaluări
- Aalco Metals LTD Aluminium Alloy 2011 T3 Rod and Bar 3Document2 paginiAalco Metals LTD Aluminium Alloy 2011 T3 Rod and Bar 3Agung SulisÎncă nu există evaluări
- OK Grain 21.85Document1 paginăOK Grain 21.85brunizzaÎncă nu există evaluări
- Aluminium Alloy 2014A T651 Sheet and Plate: Specifications Temper TypesDocument3 paginiAluminium Alloy 2014A T651 Sheet and Plate: Specifications Temper TypesMellierÎncă nu există evaluări
- High Strength, Low Alloy Steels: Tion (Ordinary Rusting, Which Is The Most Common KindDocument7 paginiHigh Strength, Low Alloy Steels: Tion (Ordinary Rusting, Which Is The Most Common KindWilly UioÎncă nu există evaluări
- LM4 Aluminium Casting Alloy (Al - Si5Cu3) : Chemical CompositionDocument3 paginiLM4 Aluminium Casting Alloy (Al - Si5Cu3) : Chemical Compositiona.suleimanÎncă nu există evaluări
- Alloy Steels CompleteDocument85 paginiAlloy Steels CompleteSurya vsÎncă nu există evaluări
- LM6 Aluminium Casting Alloyhb11Document4 paginiLM6 Aluminium Casting Alloyhb11Nader MohamedÎncă nu există evaluări
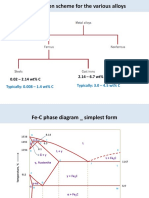
- Classification Scheme For The Various Alloys: 0.02 - 2.14 WT% C 2.14 - 6.7 WT% CDocument19 paginiClassification Scheme For The Various Alloys: 0.02 - 2.14 WT% C 2.14 - 6.7 WT% CAlex HalesÎncă nu există evaluări
- W6 Lecture 6.surface Hardening of Steel PDFDocument28 paginiW6 Lecture 6.surface Hardening of Steel PDFYota KimireÎncă nu există evaluări
- DSM-0375.0 FeMoC CompDocument3 paginiDSM-0375.0 FeMoC CompApichitÎncă nu există evaluări
- Material Chapter11Document30 paginiMaterial Chapter11khanh phamÎncă nu există evaluări
- 054 305 Descriptive Statistics - CDocument43 pagini054 305 Descriptive Statistics - CAnonymous 7yN43wjlÎncă nu există evaluări
- Turnare BetonDocument1 paginăTurnare BetonAnonymous 7yN43wjlÎncă nu există evaluări
- MSA Gage Repeatability and Reproducability: DefineDocument32 paginiMSA Gage Repeatability and Reproducability: DefineAnonymous 7yN43wjlÎncă nu există evaluări
- 335 Capability Analysis - BDocument38 pagini335 Capability Analysis - BAnonymous 7yN43wjlÎncă nu există evaluări
- 115 MSA Kappa - BDocument9 pagini115 MSA Kappa - BAnonymous 7yN43wjlÎncă nu există evaluări
- Corporate Training AS 63Document32 paginiCorporate Training AS 63Anonymous 7yN43wjlÎncă nu există evaluări
- Overview of Metal Forming ProcessesDocument19 paginiOverview of Metal Forming ProcessesAnonymous 7yN43wjlÎncă nu există evaluări
- Module 10 - Introduction To NDE2Document33 paginiModule 10 - Introduction To NDE2Anonymous 7yN43wjlÎncă nu există evaluări
- 053 300 Data Types - BDocument13 pagini053 300 Data Types - BAnonymous 7yN43wjlÎncă nu există evaluări
- HIP Process Overview: Expected AdvantagesDocument13 paginiHIP Process Overview: Expected AdvantagesAnonymous 7yN43wjlÎncă nu există evaluări
- Module 9 - WeldingDocument29 paginiModule 9 - WeldingAnonymous 7yN43wjlÎncă nu există evaluări
- Manualul de Electrice 2010Document553 paginiManualul de Electrice 2010imirescu_2Încă nu există evaluări
- 8D Problem Solving Process: Houston, We Have A ProblemDocument53 pagini8D Problem Solving Process: Houston, We Have A ProblemAnonymous 7yN43wjlÎncă nu există evaluări
- Module 6b - Casting Process1Document14 paginiModule 6b - Casting Process1Anonymous 7yN43wjlÎncă nu există evaluări
- Module 1a - Basics IDocument20 paginiModule 1a - Basics IAnonymous 7yN43wjlÎncă nu există evaluări
- Module 5-Mechanical Properties RomaniaDocument23 paginiModule 5-Mechanical Properties RomaniaAnonymous 7yN43wjlÎncă nu există evaluări
- Module 2 - Making MetalsDocument14 paginiModule 2 - Making MetalsAnonymous 7yN43wjlÎncă nu există evaluări
- Module 2 - Making MetalsDocument14 paginiModule 2 - Making MetalsAnonymous 7yN43wjlÎncă nu există evaluări
- Module 3 - Heat TreatingDocument26 paginiModule 3 - Heat TreatingAnonymous 7yN43wjlÎncă nu există evaluări
- Stas 9989 - 74 Aparate Cu Sursa Incorporata - NuclearaDocument6 paginiStas 9989 - 74 Aparate Cu Sursa Incorporata - NuclearaAnonymous 7yN43wjlÎncă nu există evaluări
- Estimation of Welding Cost: by K.R.Prasanna Venkatesan WE0663Document41 paginiEstimation of Welding Cost: by K.R.Prasanna Venkatesan WE0663Anonymous 7yN43wjl100% (1)
- Module 1b - Basics IIDocument15 paginiModule 1b - Basics IIAnonymous 7yN43wjlÎncă nu există evaluări
- Module 4a - Carbon Alloy SteelsDocument26 paginiModule 4a - Carbon Alloy SteelsAnonymous 7yN43wjlÎncă nu există evaluări
- Electric System For Houses 2Document240 paginiElectric System For Houses 2Anonymous 7yN43wjlÎncă nu există evaluări
- Instalatii Electrice 1Document234 paginiInstalatii Electrice 1Anonymous 7yN43wjl0% (1)
- ZETTLER Consultants Guide APACDocument180 paginiZETTLER Consultants Guide APACYashveer TakooryÎncă nu există evaluări
- Siemens Planning Guide E432d PDFDocument98 paginiSiemens Planning Guide E432d PDFShashish AshuÎncă nu există evaluări
- Siemens Planning Guide E432d PDFDocument98 paginiSiemens Planning Guide E432d PDFShashish AshuÎncă nu există evaluări
- José Guadalupe PosadaDocument19 paginiJosé Guadalupe PosadaJudy Baca100% (1)
- 3rd Quarter SUMMATIVE TEST in MAPEHDocument3 pagini3rd Quarter SUMMATIVE TEST in MAPEHzaile felineÎncă nu există evaluări
- DTS Nozzles R3Document2 paginiDTS Nozzles R3meilia teknikÎncă nu există evaluări
- Augmentation of Labour: Nabhan A, Boulvain MDocument8 paginiAugmentation of Labour: Nabhan A, Boulvain MMade SuryaÎncă nu există evaluări
- Thom22e ch03 FinalDocument44 paginiThom22e ch03 FinalDionisius AlvianÎncă nu există evaluări
- Kobelco CK1100G Spec BookDocument38 paginiKobelco CK1100G Spec BookEjeantengÎncă nu există evaluări
- January 11, 2019 Grade 1Document3 paginiJanuary 11, 2019 Grade 1Eda Concepcion PalenÎncă nu există evaluări
- Interection 2 Reading Teacher's Book PDFDocument165 paginiInterection 2 Reading Teacher's Book PDFتركي الزهراني0% (1)
- Paso de Blas Lying in Clinic For NewDocument5 paginiPaso de Blas Lying in Clinic For NewNaheed Dean MustafaÎncă nu există evaluări
- Quizlet Table 7Document1 paginăQuizlet Table 7JosielynÎncă nu există evaluări
- Massey Ferguson MF7600 Technician Workshop ManualDocument798 paginiMassey Ferguson MF7600 Technician Workshop Manualgavcin100% (5)
- Cinnamon RollDocument1 paginăCinnamon RollMaria Manoa GantalaÎncă nu există evaluări
- 2005 Warehouse Benchmark in GR PTDocument59 pagini2005 Warehouse Benchmark in GR PTMarco Antonio Oliveira NevesÎncă nu există evaluări
- m07srt Lesson KmarlinkDocument3 paginim07srt Lesson Kmarlinkapi-515106812Încă nu există evaluări
- Srinivasa Ramanujan - Britannica Online EncyclopediaDocument2 paginiSrinivasa Ramanujan - Britannica Online EncyclopediaEvariste MigaboÎncă nu există evaluări
- Check List For Design Program of A Parish ChurchDocument11 paginiCheck List For Design Program of A Parish ChurchQuinn HarloweÎncă nu există evaluări
- (IGC 2024) 2nd Circular - 0630Document43 pagini(IGC 2024) 2nd Circular - 0630VictoriaÎncă nu există evaluări
- Light Dimmer CircuitsDocument14 paginiLight Dimmer CircuitskapilasriÎncă nu există evaluări
- WebMethods System Requirements 8xDocument7 paginiWebMethods System Requirements 8xmaxprinceÎncă nu există evaluări
- List of Famous Cities On River Banks in The WorldDocument2 paginiList of Famous Cities On River Banks in The WorldDiptangshu DeÎncă nu există evaluări
- Epidemiological Triad of HIV/AIDS: AgentDocument8 paginiEpidemiological Triad of HIV/AIDS: AgentRakib HossainÎncă nu există evaluări
- STS Reviewer FinalsDocument33 paginiSTS Reviewer FinalsLeiÎncă nu există evaluări
- TEFL Entrance ExamDocument3 paginiTEFL Entrance ExammerekÎncă nu există evaluări
- Wind LoadingDocument18 paginiWind LoadingStephen Ogalo100% (1)
- Summary of The Pilot ProjectDocument46 paginiSummary of The Pilot ProjectSrinivasan JeganÎncă nu există evaluări
- Introduction To Physiotherapy PracticeDocument22 paginiIntroduction To Physiotherapy PracticejÎncă nu există evaluări
- Market Challengers StrategiesDocument19 paginiMarket Challengers Strategiestobbob007100% (20)
- UntitledDocument17 paginiUntitledSedat100% (1)
- Dphs Freshman Course List 2024-25Document1 paginăDphs Freshman Course List 2024-25api-337771649Încă nu există evaluări
- 30xa 100t PDFDocument162 pagini30xa 100t PDFleung ka kitÎncă nu există evaluări