Documente Academic
Documente Profesional
Documente Cultură
CA Emergency: Tumor Lysis Syndrome
Încărcat de
Jai - HoDescriere originală:
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
CA Emergency: Tumor Lysis Syndrome
Încărcat de
Jai - HoDrepturi de autor:
Formate disponibile
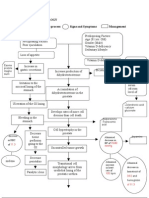
Medical Management
The potential severity of complications resulting from the development of TLS necessitates measures for prevention in high-risk patients and prompts treatment in the event that symptoms arise. Recognition of risk factors, close monitoring of at risk-patients and appropriate intervention are the key to preventing or managing tumor lysis syndrome. A cardinal sign is hyperuricemia, leading to uric acid nephropathy. Other signs are hyperkalemia, hyperphosphatemia and secondary hypocalcemia.
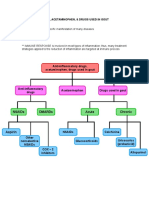
Treatment options of hyperkalemia include sodium polystyrene sulfonate (kayexalate), hypertonic glucose and insulin, diuretics (furosemide), and sodium bicarbonate (2040 mEq/L IV fluid; bolus with 0.51.0 mEq/kg for pH <6.5.) . If the patient has symptomatic hyperkalemia, the healthcare provider may order I.V. regular insulin and to redistribute potassium, shifting it intracellularly.
Hyperphosphatemia is managed with phosphate binders such as aluminum hydroxide given in limited dosages to avoid aluminum toxicity. For asymptomatic hyperphosphatemia, initial treatment consists of eliminating phosphate from intravenous solutions, maintaining adequate IV hydration because it maintains renal blood flow and promotes urinary excretion of uric acid and phosphate. , and the administration of phosphate binders. Treatment of hyperphosphatemia reduces dietary phosphate intake and includes phosphate binders such as aluminum hydroxide and aluminum carbonate.
When recurrent hypocalcemia is present, a continuous intravenous infusion of calcium gluconate can be initiated. Care must be taken because increased calcium might increase the risk of calcium phosphate precipitation in the tissues and consequential obstructive uropathy. Hypocalcemia should be managed by first treating hyperphosphatemia. If the hypocalcemia does not resolve and he patient is experiencing seizures or cardiac symptoms, seizures precautions and IV calcium gluconate are
Antihyperuricemic agents: Allopurinol -One approach to preventing or managing TLS associated hyperuricemia is to block the conversion of xanthine and hypoxanthine to uric acid. Allopurinol is a xanthine analog which, when converted in vivo to oxypurinol, acts as a competitive inhibitor of xanthine oxidase, thereby blocking the conversion of the purine metabolites to uric acid. -Use of Allopurinol has been shown to decrease the formation of uric acid and to reduce the incidence of obstructiove uropathy caused by uric acid precipitation in patients at risk for developing TLS.
Recombinant urate oxidase(rasburicase) -Rasburicase is administered for 5 to 7 days, and chemotheraphy could be started 4h after initiation of treatment. A recombinant urate oxidase enzyme, rasburicase converts existing uric acid to allantoin, which is 5 to 10 times more soluble in urine than uric acid. Rasburicase differs from allopurinol since it can affect existing plasma uric acid; allopurinol affects only the future production of uric acid by inhibiting
REMEMBER!
Alkalinization is recommended during allopurinol treatment, but is not required with rasburicase because there was a controversy before that alkalinization may increase precipitation of calcium phosphate in renal tubules; it also interferes with renal tubular reabsorption of phosphorus.
Fluid
and hydrations
-Hydrations is the first and most important line of prevention. IV fluid should be 3000 mL/m/24 h or more(amount may vary depending on age and comorbidities) and continued for several days to maintain a urine output of more than 100 cc/m/hour and urine specific gravity of less than 1.010. It is especially important to work with physicians and residents to ensure that the patient receives no additional potassium during hydration, even if the patients potassium level is normal, because the patient will be exposed to an elevated level of potassium when lysis begins.
Additional Treatment
Hemofiltration/dialysis
S-ar putea să vă placă și
- Biological ChemistryDocument28 paginiBiological ChemistryArsilan Aziz LoneÎncă nu există evaluări
- GoutDocument6 paginiGoutNader Smadi100% (1)
- The Hitchhiker's Guide To Clinical Pharmacology: Pharmacodynamics: How Drugs WorkDocument23 paginiThe Hitchhiker's Guide To Clinical Pharmacology: Pharmacodynamics: How Drugs WorkMylz MendozaÎncă nu există evaluări
- Philippine Constitution: Article VII Section 17, 18, 19Document29 paginiPhilippine Constitution: Article VII Section 17, 18, 19Jai - Ho95% (22)
- Asking Your Question (PICO) - NursingDocument5 paginiAsking Your Question (PICO) - NursingBentaigaÎncă nu există evaluări
- Acute Glomrulonephritis PathophysiologyDocument2 paginiAcute Glomrulonephritis PathophysiologyJai - HoÎncă nu există evaluări
- Diabetes PathoDocument2 paginiDiabetes Pathodrewcel100% (1)
- Tumor Lysis SyndromeDocument35 paginiTumor Lysis SyndromeJai - HoÎncă nu există evaluări
- Fdar 3Document17 paginiFdar 3leslie_macasaet75% (4)
- Acute Respiratory Distress SyndromeDocument30 paginiAcute Respiratory Distress SyndromeOya Zuraini KamalÎncă nu există evaluări
- Color and Art TherapyDocument30 paginiColor and Art TherapyJai - Ho100% (1)
- NSAIDsDocument12 paginiNSAIDsjelly100% (1)
- Acute Coronary Syndrome, STEMI, Anterior Wall, Killips - 1, DM Type II - UncontrolledDocument61 paginiAcute Coronary Syndrome, STEMI, Anterior Wall, Killips - 1, DM Type II - UncontrolledJai - Ho100% (5)
- NCP On Electrolyte ImbalanceDocument4 paginiNCP On Electrolyte Imbalancefreyah_bc67% (3)
- NCP On Acute GlumrulonephritisDocument11 paginiNCP On Acute GlumrulonephritisJai - Ho89% (9)
- Community Acquired Pneumonia, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsDe la EverandCommunity Acquired Pneumonia, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsÎncă nu există evaluări
- Case Study On Acute GlomerulonephritisDocument20 paginiCase Study On Acute GlomerulonephritisJai - Ho87% (15)
- Case Study Liver CirrhosisDocument64 paginiCase Study Liver CirrhosisJoseph Emmanuel CandaÎncă nu există evaluări
- Moh Exam Prep MaterialDocument159 paginiMoh Exam Prep MaterialAbid Ali Khan100% (1)
- Di, Siadh, CSWDocument17 paginiDi, Siadh, CSWNyomanGinaHennyKristiantiÎncă nu există evaluări
- Cancer ChemotherapyDocument9 paginiCancer ChemotherapyJennicaÎncă nu există evaluări
- GoutDocument10 paginiGoutAmberÎncă nu există evaluări
- Case Study On Prostatic CancerDocument21 paginiCase Study On Prostatic CancerJai - Ho100% (3)
- Medical Genetics SummariesDocument462 paginiMedical Genetics SummariesMohd RahimiÎncă nu există evaluări
- Nursing Care Plan: Clustered Cues Nursing Diagnosis Rationale Outcome Criteria Nursing Interventions Rationale EvaluationDocument2 paginiNursing Care Plan: Clustered Cues Nursing Diagnosis Rationale Outcome Criteria Nursing Interventions Rationale EvaluationKarl Lourenz Deysolong100% (1)
- A Case Study On Rectal AdenocarcinomaDocument62 paginiA Case Study On Rectal AdenocarcinomaEyySiEffVee100% (1)
- Allopurinol Drug StudyDocument2 paginiAllopurinol Drug StudyHailMarieSBarcenasÎncă nu există evaluări
- Course in The WardDocument4 paginiCourse in The Wardkingkiel50% (2)
- Lab 5 Diabetes InsipidusDocument6 paginiLab 5 Diabetes InsipidusLisa EkapratiwiÎncă nu există evaluări
- Case Study 2Document4 paginiCase Study 2api-2451636590% (1)
- Leptospirosis: Diagnosis & TreatmentDocument20 paginiLeptospirosis: Diagnosis & TreatmentMuhammed SalahÎncă nu există evaluări
- Dr. Sunatrio - Management Hypovolemic ShockDocument59 paginiDr. Sunatrio - Management Hypovolemic ShockArga Putra SaboeÎncă nu există evaluări
- Venous Thromboembolism (VTE) - McMaster Pathophysiology ReviewDocument9 paginiVenous Thromboembolism (VTE) - McMaster Pathophysiology ReviewFadiyah UlfahÎncă nu există evaluări
- Example Case StudyDocument7 paginiExample Case StudyTerrena Lyn BlackmanÎncă nu există evaluări
- Failure To ThriveDocument3 paginiFailure To Thriveibbs91Încă nu există evaluări
- Pharmacologic Management: BleomycinDocument1 paginăPharmacologic Management: BleomycinKim ApuradoÎncă nu există evaluări
- Pleural EffusionDocument12 paginiPleural EffusionWan HafizÎncă nu există evaluări
- MI LABS ExplainedDocument3 paginiMI LABS Explainedjrubin83669Încă nu există evaluări
- Case Study Bacterial MeningitisDocument5 paginiCase Study Bacterial MeningitisChristine SaliganÎncă nu există evaluări
- Case PresentationDocument6 paginiCase PresentationYrrem UbaganÎncă nu există evaluări
- Pathophysiology of Peptic Ulcer DiseaseDocument1 paginăPathophysiology of Peptic Ulcer DiseaseJhade RelletaÎncă nu există evaluări
- DB31 - Pathophysiology of Diabetes Mellitus and HypoglycemiaDocument5 paginiDB31 - Pathophysiology of Diabetes Mellitus and HypoglycemiaNeil Alcazaren かわいいÎncă nu există evaluări
- AMLDocument19 paginiAMLquerokeropi100% (1)
- Chronic PyelonephritisDocument5 paginiChronic PyelonephritisIsak ShatikaÎncă nu există evaluări
- Carbon Monoxide Poisoning in ChildrenDocument10 paginiCarbon Monoxide Poisoning in ChildrenSubash PaudealÎncă nu există evaluări
- Pathophysiology of Portal HYPERTENSION PDFDocument11 paginiPathophysiology of Portal HYPERTENSION PDFCamilo VidalÎncă nu există evaluări
- Kaposi's SarcomaDocument6 paginiKaposi's SarcomaveremkovichÎncă nu există evaluări
- AcknowledgementDocument9 paginiAcknowledgementjhzenÎncă nu există evaluări
- Anaphylactic ReactionDocument9 paginiAnaphylactic ReactionZahir Jayvee Gayak IIÎncă nu există evaluări
- Pulmonary EmbolismDocument16 paginiPulmonary EmbolismniyigokÎncă nu există evaluări
- Case Presentation: Patient Chart - Mary JohnsonDocument12 paginiCase Presentation: Patient Chart - Mary Johnsonivoneeh_16100% (1)
- Approach To Pleural EffusionDocument46 paginiApproach To Pleural EffusionBaskoro Tri LaksonoÎncă nu există evaluări
- Vincristine MonographDocument9 paginiVincristine MonographcmeytasÎncă nu există evaluări
- MedSurg Notes - Cancer of The LiverDocument2 paginiMedSurg Notes - Cancer of The LiverMae CeaesarÎncă nu există evaluări
- Liver Cirrhosis Care PlanDocument3 paginiLiver Cirrhosis Care PlanWendy EscalanteÎncă nu există evaluări
- Fasting Blood Glucose TestDocument10 paginiFasting Blood Glucose TestBrylle ArbasÎncă nu există evaluări
- Cerebrovascular Disease (Bleed)Document25 paginiCerebrovascular Disease (Bleed)Margaret Jenaw JenawÎncă nu există evaluări
- BSN4D-SG2 DM Type2Document201 paginiBSN4D-SG2 DM Type2Charisse CaydanÎncă nu există evaluări
- Ms Test-Questio 3Document7 paginiMs Test-Questio 3Jackie AbarraÎncă nu există evaluări
- Relapsed Refractory AMLDocument114 paginiRelapsed Refractory AMLKishoreChandraKoradaÎncă nu există evaluări
- NCP CvaDocument4 paginiNCP CvaMariquita BuenafeÎncă nu există evaluări
- Minggu 5 LP THALASSEMIADocument14 paginiMinggu 5 LP THALASSEMIAMuhammad PanduÎncă nu există evaluări
- Thyroid Papillary Carcinoma CaseDocument6 paginiThyroid Papillary Carcinoma CaseRandy F BabaoÎncă nu există evaluări
- Inflammatory Bowel Disease .. Last EditDocument22 paginiInflammatory Bowel Disease .. Last EditRashed ShatnawiÎncă nu există evaluări
- ESRD PathophysiologyDocument2 paginiESRD Pathophysiologynursing concept mapsÎncă nu există evaluări
- Pahtophysiology of EsrdDocument5 paginiPahtophysiology of EsrdCarl JardelezaÎncă nu există evaluări
- Epidemiology, Pathogenesis, and Pathology of NeuroblastomaDocument21 paginiEpidemiology, Pathogenesis, and Pathology of NeuroblastomaHandre PutraÎncă nu există evaluări
- Sick Sinus Syndrome 1Document23 paginiSick Sinus Syndrome 1Salman HabeebÎncă nu există evaluări
- Type 1 Diabetes Mellitus - Practice Essentials, Background, PathophysiologyDocument8 paginiType 1 Diabetes Mellitus - Practice Essentials, Background, PathophysiologyTrifosa Ika Septiana EryaniÎncă nu există evaluări
- Lupus NephritisDocument15 paginiLupus NephritisVilza maharaniÎncă nu există evaluări
- Head Nurse Experience (Staffing)Document3 paginiHead Nurse Experience (Staffing)Abigail BrillantesÎncă nu există evaluări
- Cholecystectomy (: Laparoscopic GallstonesDocument4 paginiCholecystectomy (: Laparoscopic GallstonesAlexia BatungbacalÎncă nu există evaluări
- FlashPath - Lung - Bronchopulmonary DysplasiaDocument15 paginiFlashPath - Lung - Bronchopulmonary DysplasiaHazem AliÎncă nu există evaluări
- Mallory Weiss SyndromeDocument10 paginiMallory Weiss SyndromeGuilherme CaneverÎncă nu există evaluări
- The Difference Between Toxic and Nontoxic GoiterDocument2 paginiThe Difference Between Toxic and Nontoxic GoiterJawad Rehman100% (1)
- Fludrocortisone (Florinef)Document17 paginiFludrocortisone (Florinef)passer byÎncă nu există evaluări
- Schematic Diagram of StrokeDocument1 paginăSchematic Diagram of StrokeMaricar K. BrionesÎncă nu există evaluări
- Phosphate BinderDocument51 paginiPhosphate BinderbedestySÎncă nu există evaluări
- HyperkalaemiaDocument1 paginăHyperkalaemiaRekaBÎncă nu există evaluări
- Laporan Kasus TLS (Tumor Lisis Sindrome)Document58 paginiLaporan Kasus TLS (Tumor Lisis Sindrome)Petrus IriantoÎncă nu există evaluări
- Care StudyDocument10 paginiCare StudyJai - HoÎncă nu există evaluări
- IVF ProblemsDocument1 paginăIVF ProblemsJai - HoÎncă nu există evaluări
- Final Essay JADocument4 paginiFinal Essay JAJai - HoÎncă nu există evaluări
- CA Emergency: Tumor Lysis SyndromeDocument10 paginiCA Emergency: Tumor Lysis SyndromeJai - HoÎncă nu există evaluări
- Drugs Project in Ms.Document5 paginiDrugs Project in Ms.Jai - HoÎncă nu există evaluări
- Final - Spinal Stenosis L4, L5 Secondary To Spondylolisthesis L4, L5 Grade II With Hypertrophized Ligament Um and Radiculopathy With Myelopathy Right SidedDocument66 paginiFinal - Spinal Stenosis L4, L5 Secondary To Spondylolisthesis L4, L5 Grade II With Hypertrophized Ligament Um and Radiculopathy With Myelopathy Right SidedJai - Ho100% (1)
- Agn Patho Repaired)Document3 paginiAgn Patho Repaired)Jai - HoÎncă nu există evaluări
- Fundamentals of MedicationDocument18 paginiFundamentals of MedicationJai - Ho100% (1)
- Dissecting The Nature of The Silent Assailant - FINALDocument77 paginiDissecting The Nature of The Silent Assailant - FINALJai - HoÎncă nu există evaluări
- Left Thyroid Papillary Carcinoma, Status Post Fine Needle Biopsy Stage 1 T2N0MODocument55 paginiLeft Thyroid Papillary Carcinoma, Status Post Fine Needle Biopsy Stage 1 T2N0MOJai - HoÎncă nu există evaluări
- Case Study Final. Pott's DiseaseDocument50 paginiCase Study Final. Pott's DiseaseJaiRus MagdadaRo100% (3)
- Mine - Assignment in RLE50Document54 paginiMine - Assignment in RLE50Jai - HoÎncă nu există evaluări
- Pa Tho of Prostatic CancerDocument2 paginiPa Tho of Prostatic CancerJai - HoÎncă nu există evaluări
- Drug Order For Prostatic CancrDocument11 paginiDrug Order For Prostatic CancrJai - HoÎncă nu există evaluări
- Vii. Nursing Care Plan: Secretions in The AirwaysDocument5 paginiVii. Nursing Care Plan: Secretions in The AirwaysJai - Ho100% (2)
- Acute Glomerulonephritis Complicated UTIDocument13 paginiAcute Glomerulonephritis Complicated UTIJai - HoÎncă nu există evaluări
- Final Lab - Results of Patient Who Has Electrolyte ImbalanceDocument7 paginiFinal Lab - Results of Patient Who Has Electrolyte ImbalanceJai - HoÎncă nu există evaluări
- Lab - Results On Acute GlomerulonephritisDocument16 paginiLab - Results On Acute GlomerulonephritisJai - Ho100% (1)
- Possible Drugs of Acute GlomerulonephritisDocument10 paginiPossible Drugs of Acute GlomerulonephritisJai - HoÎncă nu există evaluări
- Nahco3 AllopDocument3 paginiNahco3 AllopKurtt Evan ValinoÎncă nu există evaluări
- APNC 520 Exam 10 Study Guide Musculoskeletal DisordersDocument15 paginiAPNC 520 Exam 10 Study Guide Musculoskeletal DisordersMariaÎncă nu există evaluări
- Ify Drug StudiesDocument15 paginiIfy Drug StudiesifyÎncă nu există evaluări
- Classification of Drug Hypersensitivity Into Allergic, P-I, and Pseudo-Allergic FormsDocument14 paginiClassification of Drug Hypersensitivity Into Allergic, P-I, and Pseudo-Allergic FormsFanny PritaningrumÎncă nu există evaluări
- Anticancer Drugs NotesDocument112 paginiAnticancer Drugs Notesdnyanshri bhamareÎncă nu există evaluări
- Metabolism of Nucleoproteins Part IDocument50 paginiMetabolism of Nucleoproteins Part IAgafioÎncă nu există evaluări
- 2021 Oct Dwe Adult Medicine Practice Exam Questions and AnswersDocument18 pagini2021 Oct Dwe Adult Medicine Practice Exam Questions and AnswersMohammed HamedÎncă nu există evaluări
- Complete Drug StudyDocument239 paginiComplete Drug StudyRPh Krishna Chandra Jagrit0% (1)
- Office of The Infection Control Committee: Allied Care Experts (Ace) Medical Center - PaterosDocument5 paginiOffice of The Infection Control Committee: Allied Care Experts (Ace) Medical Center - PaterosCris GalendezÎncă nu există evaluări
- AllopurinolDocument7 paginiAllopurinolSahera Nurhidayah NasutionÎncă nu există evaluări
- Fphar 11 578318Document24 paginiFphar 11 578318ronakamilah_8307Încă nu există evaluări
- Tumor Lysis SyndromeDocument14 paginiTumor Lysis SyndromejesusÎncă nu există evaluări
- Non-Protein Nitrogenous Constituents of Blood - Urea, Uric Acid EtcDocument50 paginiNon-Protein Nitrogenous Constituents of Blood - Urea, Uric Acid EtcBobskinnyÎncă nu există evaluări
- GoutDocument47 paginiGoutsyifasqaÎncă nu există evaluări
- Obat Induksi HIPERURISEMIA A PDFDocument10 paginiObat Induksi HIPERURISEMIA A PDFshelfinararaÎncă nu există evaluări
- Med Surg4Document27 paginiMed Surg4Hasan A AsFourÎncă nu există evaluări
- Febuxostat in GoutDocument7 paginiFebuxostat in GoutLia NadaÎncă nu există evaluări
- MCQ Sample Test Paper-Dotes PharmaDocument7 paginiMCQ Sample Test Paper-Dotes PharmaDominus TestimoniumÎncă nu există evaluări
- Diagnosis Dan Talak Gout: DR - Dr. Asep Sukohar, M.Kes Bagian Farmakologi Dan Terapi FK UnilaDocument26 paginiDiagnosis Dan Talak Gout: DR - Dr. Asep Sukohar, M.Kes Bagian Farmakologi Dan Terapi FK UnilaArifah Afkar FadilahÎncă nu există evaluări